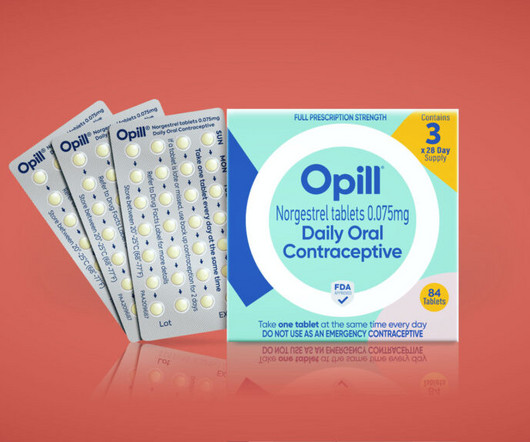
STAT+: How the first over-the-counter birth control pill in the U.S. got its ‘unapologetic’ design
STAT
JULY 27, 2023
It’s not every day that a marketing team is tasked with designing the branding and packaging of the first over-the-counter birth control pill to be sold in the U.S.












































Let's personalize your content