Microbial burden assessment of solid pharmaceutical products
European Pharmaceutical Review
APRIL 19, 2024
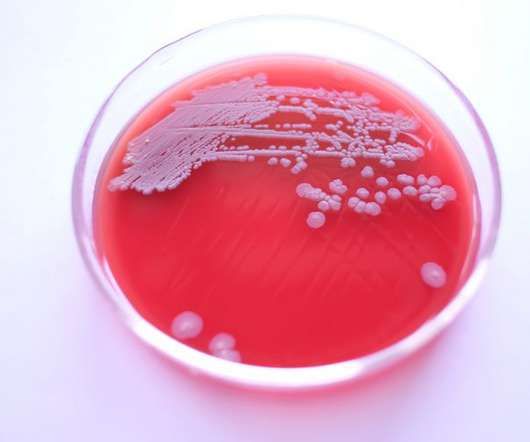
The “accurate, precise, and robust” data obtained via the dew point chilled mirror method, means it is an “outstanding” approach for quantifying water activity status in tablets and capsules, according to a paper published in RPS Pharmacy and Pharmacology Reports. coli in tablets and capsules with water activity lower than 0.60”.











































Let's personalize your content