Dangerous data delays – a real-time problem
pharmaphorum
SEPTEMBER 16, 2020
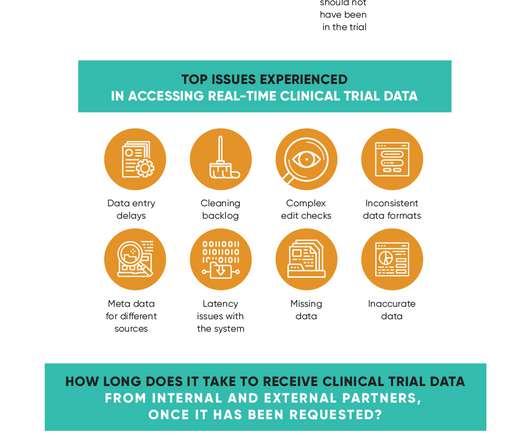
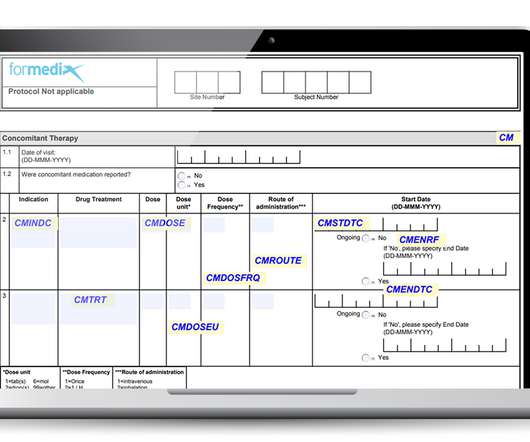
Delayed clinical trial data delivery is a growing problem for pharmaceutical companies and one that can have catastrophic consequences for the development of their promising pipeline candidates. But there are ways to improve this situation, as Remarque Systems’ recent Is a lack of real-time data holding trials back?










Let's personalize your content