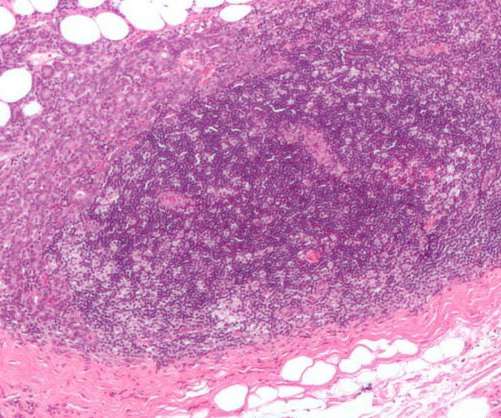
Japan’s MHLW approves Daiichi Sankyo’s breast cancer treatment
Pharmaceutical Technology
NOVEMBER 25, 2022
The treatment is indicated for usage in such patients following previous chemotherapy, comprising trastuzumab and a taxane. Nausea, fatigue, vomiting, reduced neutrophil count, alopecia and anaemia among others were observed to be the most prevalent adverse reactions.













Let's personalize your content