US FDA approves Incyte’s merkel cell carcinoma therapy
Pharmaceutical Technology
MARCH 23, 2023
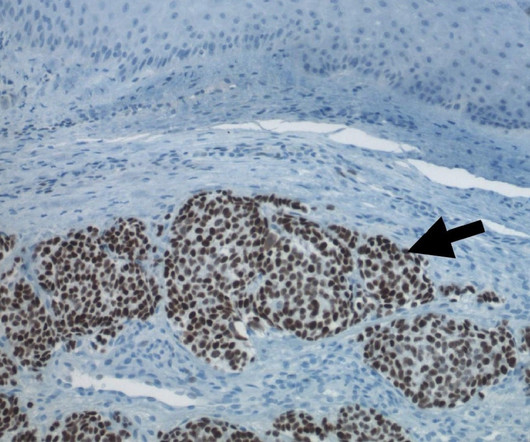
The US Food and Drug Administration (FDA) has approved Incyte ’s Zynyz (retifanlimab-dlwr) to treat metastatic or recurrent locally advanced merkel cell carcinoma (MCC) in adult patients. It is also being assessed in other tumour types and along with other Incyte pipeline compounds.







Let's personalize your content