JPM24: Riding label expansions and launches, AZ plots course toward 'industry-leading growth' by 2030
Fierce Pharma
JANUARY 10, 2024
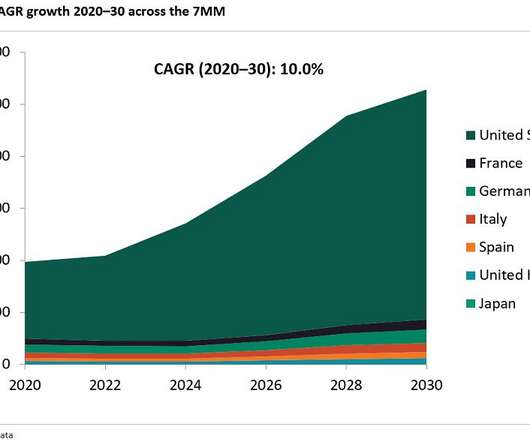
In AZ's push to reach industry-leading growth by 2030, confidence is key, chief financial officer Aradhana Sarin said at the J.P. With a late-stage pipeline spanning more than 120 clinical trials and a firm grasp of five major disease areas, AstraZeneca appears confident heading into 2024. | Morgan Healthcare Conference.


















Let's personalize your content