AstraZeneca acquires TeneoTwo in $1.3bn deal to broaden haematology portfolio
Pharmaceutical Technology
JULY 8, 2022
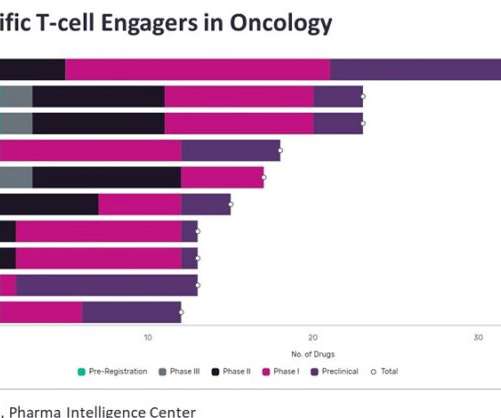
In December 2014, Amgen’s landmark FDA approval saw Blincyto (blinatumomab) approved for relapsed/refractory (R/R) B-cell progenitor acute lymphoblastic leukaemia (B-ALL), at the time being the only marketed BiTE. Currently, there are 110 BiTEs in oncology clinical trials, with seven in Phase III and three in pre-registration. months vs. 4.0









Let's personalize your content