STAT+: Medicare expects to spend $3.5 billion on new Alzheimer’s drug in 2025
STAT
APRIL 11, 2024
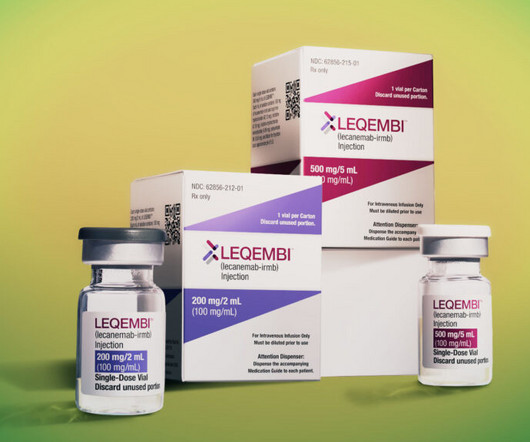
Medicare for the first time has estimated that a new Alzheimer’s treatment could cost the program billions of dollars by next year — well beyond what Wall Street or even the drug’s manufacturer have projected — according to a document obtained by STAT.




































Let's personalize your content