FDA Approves Updated Label for Altuviiio for Effective Bleed Protection in Children Younger than 12 Years
Drug Topics
MAY 13, 2024
Altuviiio was first granted approval by the FDA in February 2023.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.
Drug Topics
MAY 13, 2024
Altuviiio was first granted approval by the FDA in February 2023.

pharmaphorum
NOVEMBER 17, 2023
AZ first to AKT finish line, but FDA clears narrow label Phil.Taylor Fri, 17/11/2023 - 09:46 Bookmark this
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pharmacy Times
MAY 13, 2024
Antihemophilic factor (recombinant) Fc-VWF-XTEN fusion protein-ehtl was initally approved in February 2023 for adults and children with hemophilia A for prophylaxis and on-demand treatment to control bleeding.

The FDA Law Blog
DECEMBER 8, 2022
Food and Drug Administration (FDA) issued two guidance documents, one draft and one final, on food allergen labeling requirements. 1, 2023; The applicability of food allergen labeling requirements to specific products (e.g., 1, 2023; The applicability of food allergen labeling requirements to specific products (e.g.,

The FDA Law Blog
AUGUST 21, 2023
Livornese — I saw the sign…and the answer is no—FDA-approved labeling apparently is not enough under state failure-to-warn laws, according to certain courts.
Pharmaceutical Technology
APRIL 17, 2023
The US Food and Drug Administration (FDA) has approved an update to the indications and usage section of Horizon Therapeutics ’ Tepezza (teprotumumab-trbw) label to specify its use to treat thyroid eye disease (TED) patients regardless of disease activity or duration. The FDA’s approval was granted in January 2020.

The FDA Law Blog
AUGUST 17, 2023
Tobolowsky — In January 2023, Vanda Pharmaceuticals, Inc. FDA-2023-P-0313 and FDA-2023-P-0344 ) regarding its product Hetlioz (tasimelteon). FDA regulations, at 21 C.F.R. a)(8)(iv), interpret these provisions to also allow changes due to an aspect of labeling protected by patent or exclusivity.

The FDA Law Blog
MAY 23, 2023
Food and Drug Administration (FDA) released a draft update to its Compliance Policy Guide (CPG) for FDA staff on the Agency’s enforcement of major food allergen labeling and cross-contact. The draft CPG directs FDA field staff to examine possible food product adulteration due to labeling related to allergen cross-contact.

The Checkup by Singlecare
NOVEMBER 9, 2023
Food and Drug Administration (FDA) just approved Zepbound (tirzepatide) for chronic weight management. The injectable medication is a new version of Eli Lilly’s Mounjaro, which is approved by the FDA to control blood sugar in people with Type 2 diabetes. Zepbound, on the other hand, has been FDA-approved for weight loss.

The FDA Law Blog
JANUARY 2, 2023
Hosted by American Conference Institute, the FDA Boot Camp returns for its 40th iteration with the continued intent of providing an essential working knowledge of core FDA concepts, and real-world examples that will help you to excel in your everyday practices. Labeling in the drug and biologics approval process.

The FDA Law Blog
MARCH 29, 2023
Richardson — Early on March 29, 2023, FDA announced the landmark approval of Narcan (naloxone hydrochloride) Nasal Spray for use as a nonprescription opioid overdose reversal agent. FDA Commissioner Robert M. According to this announcement, FDA approval of RiVive is anticipated in July 2023 and the U.S.

The FDA Law Blog
FEBRUARY 16, 2023
Richardson — On February 15, 2023, the Nonprescription Drugs Advisory Committee (NDAC) and the Anesthetic and Analgesic Drug Products Advisory Committee (AADPAC) held a joint meeting to discuss an application pending before FDA that would switch Narcan (naloxone) Nasal Spray from prescription to over-the-counter (OTC) status.
European Pharmaceutical Review
OCTOBER 18, 2023
The US Food and Drug Administration (FDA) has approved XPHOZAH ® (tenapanor), the first and only phosphate absorption inhibitor. XPHOZAH is expected to be available to eligible patients in the US in November 2023.

Pharmaceutical Technology
APRIL 17, 2023
On 14 April 2023, experts from the US Food and Drug Administration’s (FDA) Advisory Committee (AdCom) voted largely in favour of the potential approval of Otsuka’ s and Lundbeck Pharmaceuticals’ Rexulti for the treatment of agitation associated with Alzheimer’s dementia (AAD). Rexulti is an atypical antipsychotic.

Pharmaceutical Technology
DECEMBER 11, 2023
Novartis is seeking approval for Kisqali as a treatment of early breast cancer, with an FDA application planned by the end of 2023.

OctariusRx
MAY 15, 2023
Our 2023 nursing calculations quiz is now available. Food and Drug Administration (FDA) receives more than 100,000 reports associated with a suspected medication error. Read the medication label carefully: This a critically important step and one that often leads to errors and potential patient harm. Take the quiz and find out!
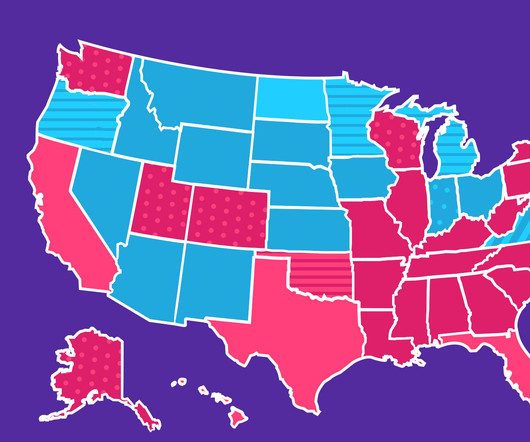
The Checkup by Singlecare
DECEMBER 5, 2023
In 2023, nine drugs made our annual list of top prescriptions by state, with vitamin D being the most filled. Keep reading to find out which medications were filled with SingleCare the most in your state in 2023. In 2022 and 2023, it was the most commonly prescribed drug in four states (versus six in 2021 ). People in the U.S.

Pharmaceutical Technology
MARCH 2, 2023
The US Food and Drug Administration (FDA) has approved Reata Pharmaceuticals ’ oral, once-daily medication SKYCLARYS (omaveloxolone) to treat Friedreich’s ataxia patients. There are three more drug candidates with major trial readouts that are expected in 2023.
Pharmaceutical Technology
MAY 29, 2023
Lexicon Pharmaceuticals (Lexicon) has received approval from the US Food and Drug Administration (FDA) for its Inpefa drug to treat heart failure. We expect this important innovation to be commercially available in the US market by the end of June 2023.”

The FDA Law Blog
JANUARY 1, 2024
Maybe that’s also why FDA last week publicized the highest number of important Warning Letters of the year (compared with prior releases in 2023). Perhaps FDA wanted us to remember 2023 as the year FDA succeeded in uncovering critical defects in drug and device manufacturing, and in critical trials.
Pharmaceutical Technology
MAY 2, 2023
The US Food and Drug Administration (FDA) has accepted the supplemental biologics licence application submitted by Bristol Myers Squibb for Reblozyl (luspatercept-aamt) as a first-line treatment of anaemia in adults with lower-risk myelodysplastic syndromes (MDS).
Pharmaceutical Technology
OCTOBER 31, 2022
The US Food and Drug Administration (FDA) has accepted Outlook Therapeutics’ Biologics License Application (BLA) filing for ONS-5010 / LYTENAVA (bevacizumab-vikg) to treat wet age-related macular degeneration (wet AMD). The regulator has set 29 August 2023 as a Prescription Drug User Fee Act (PDUFA) goal date.

pharmaphorum
AUGUST 21, 2022
Shares in Axsome Therapeutics have rocketed on FDA approval of its depression therapy Auvelity (formerly AXS-05) – a year after its approval was held up by the regulator. However, it has a broader label as unlike J&J’s drug it is indicated for use in previously-untreated MDD. Photo by Sydney Sims on Unsplash.
Pharmaceutical Technology
MAY 18, 2023
The US Food and Drug Administration (FDA) has accepted Ardelyx’s resubmission of a new drug application (NDA) for XPHOZAH (tenapanor) to control serum phosphate in adults with chronic kidney disease on dialysis who have had insufficient response or intolerance to a phosphate binder treatment.

PharmaShots
MARCH 17, 2023
5-Adapted Bivalent Booster to Treat COVID-19 in Children ≤5 Years Date: Mar 15, 2023 | Tags: Pfizer, BioNTech, Omicron BA.4/BA.5-Adapted 5-Adapted Bivalent Booster to Treat COVID-19 in Children ≤5 Years Date: Mar 15, 2023 | Tags: Pfizer, BioNTech, Omicron BA.4/BA.5-Adapted

PharmaShots
JUNE 16, 2023
Date: June 12, 2023 | Tags: Novartis, Chinook Therapeutics, Atrasentan, Zigakibart, BION-1301, IgA nephropathy, M&A, ~$3.5B Date: June 12, 2023 | Tags: Novartis, Chinook Therapeutics, Atrasentan, Zigakibart, BION-1301, IgA nephropathy, M&A, ~$3.5B
Pharmaceutical Technology
MARCH 30, 2023
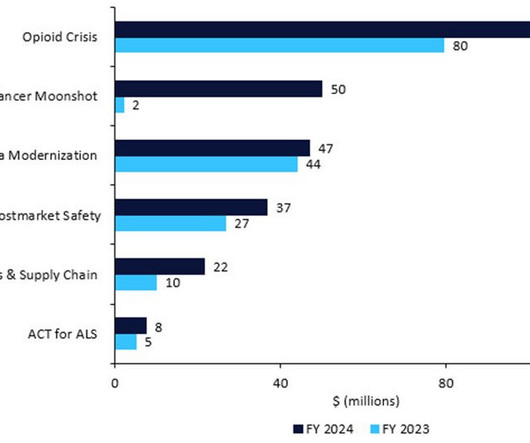
The US FDA has revealed its detailed budget proposal for FY2024, which would require pharma companies to name their active pharmaceutical ingredient (API) suppliers, restart President Biden’s Cancer Moonshot, inject cash into amyotrophic lateral sclerosis (ALS) research, and enforce stricter rules around manufacturing, recalls, and shortages.

PharmaShots
FEBRUARY 3, 2023
4D Molecular Therapeutics Receives the US FDA’s IND Clearance of 4D-150 for the Treatment of Diabetic Macular Edema Date: Feb 03, 2023 | Tags: 4D Molecular Therapeutics, 4D-150, Diabetic Macular Edema, Regulatory, US, FDA, IND AstraZeneca and Amgen Receive the US FDA’s Approval of Tezspire (tezepelumab) for the Treatment of Severe Asthma (..)

PharmaShots
APRIL 21, 2023
Oblato Reports the First Patient Enrolment of OKN-007 in the P-I Clinical Trial for Recurrent High-Grade Glioma Date: Apr 21, 2023 | Tags: Oblato, OKN-007, Recurrent High-Grade Glioma, Regulatory, Henry Ford Health System CARsgen's CT041 Receives the US FDA’s IND Clearance for the Postoperative Adjuvant Therapy of Pancreatic Cancer Date: (..)

The FDA Law Blog
JUNE 27, 2023
Walsh — Last fall, we blogged about the process FDA uses to review allegations of regulatory misconduct against device manufacturers, suggesting greater transparency on the FDA process was needed (see here ). Any comments to the public notice must be submitted by August 11, 2023. This statistic is quite disheartening.

The Checkup by Singlecare
DECEMBER 6, 2023
In 2023, medications like Ozempic , Mounjaro , and Wegovy soared in popularity—so much so that many pharmacies faced a shortage. Read on to see what readers cared about the most in 2023. Whether you’re taking Ozempic for diabetes or off-label for weight loss, you might be curious if it’s safe to mix it with alcohol.
Pharmaceutical Technology
MAY 3, 2023
PharmaTher has submitted a fast track application for Ketarx (ketamine) to the US Food and Drug Administration (FDA) to treat levodopa-induced dyskinesia in Parkinson’s disease (LID-PD). PharmaTher is now assessing the design of the Phase III trial to align with the recommendations of the FDA.
Pharmaceutical Technology
APRIL 28, 2023
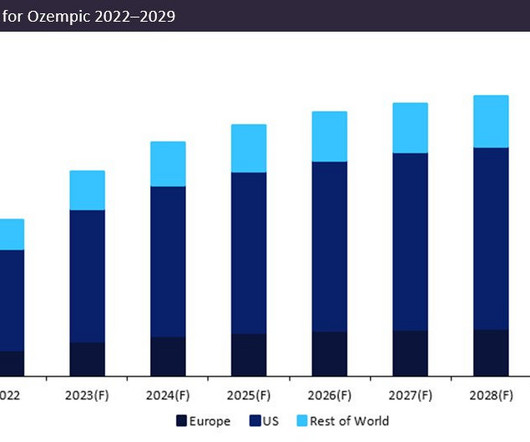
Novo Nordisk’s leading drug Ozempic (semaglutide) is forecast to demonstrate a sales growth of 23% in 2023. Ozempic’s forecast 2023 sales of $12.5bn consolidate its position as the dominant market leader, with projected sales in 2023 54% greater than closest competitor Trulicity (dulaglutide) by Eli Lilly, which anticipates sales of $8bn.
Pharmaceutical Technology
APRIL 14, 2023
The US Food and Drug Administration (FDA) has rejected Eli Lilly’s biologic licence application (BLA) for the ulcerative colitis (UC) drug mirikizumab over manufacturing concerns. No concerns related to the clinical data package, safety or the medicine label. The regulator has issued a complete response letter.
Pharmaceutical Technology
MAY 31, 2023
The US Food and Drug Administration (FDA) has accepted Bristol Myers Squibb’s new drug application (NDA) for repotrectinib for priority review. The NDA was based on data from the first-in-human, open-label, global, multi-centre TRIDENT-1 trial. Its favourable properties for penetrating the human brain increase intracranial activity.

STAT
JANUARY 25, 2024
One person also experienced hypoglycemia in 2023 after injecting a compounded version of Ozempic, said the organization, which represents 55 regional poison centers across the country and works with the U.S. Ozempic and similar diabetes medicines have been increasingly used off label for weight loss.
pharmaphorum
SEPTEMBER 30, 2022
Patient organisations have been celebrating the FDA approval yesterday of Amylyx’ amyotrophic lateral sclerosis (ALS) therapy Relyvrio, after not one but two advisory committee meetings that arrived at different conclusions about the drug. The drug was also filed for approval in Europe in February, with a decision due in early 2023.
Pharmaceutical Technology
MAY 26, 2023
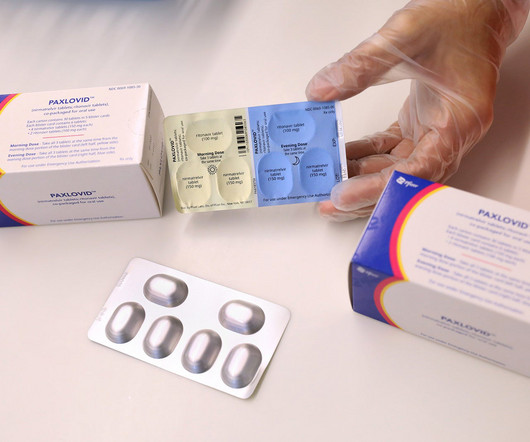
After earning multimillion dollar revenues while being an authorised preferred treatment for Covid-19, the US Food and Drug Administration (FDA) has granted a full approval to Pfizer’s oral antiviral Paxlovid (nirmatrelvir + ritonavir).

The Checkup by Singlecare
OCTOBER 4, 2023
That’s why you may have seen people talk about a medication called Vyvanse, which is FDA approved to treat ADHD and binge eating disorder, as a good option for weight loss. In 2015, the FDA approved Vyvanse to be used in the treatment of binge eating disorder. Does Vyvanse cause weight loss?

The Checkup by Singlecare
DECEMBER 1, 2023
That is 170% of the daily value (DV) recommended by the US Food and Drug Administration (FDA). Department of Agriculture (USDA) requires it to be labeled “farmed” or” wild-caught.” Check the nutrition facts label to find the amount of vitamin D and other nutrients contained in individual products. mcg or 100–144 IU per cup.

pharmaphorum
JUNE 20, 2022
Nuplazid (pimavanserin) is already FDA-approved to treat psychosis caused by Parkinson’s disease, but Acadia’s hope to expanding that use to the Alzheimer’s population were shot down by a complete response letter (CRL) from the regulator last year.
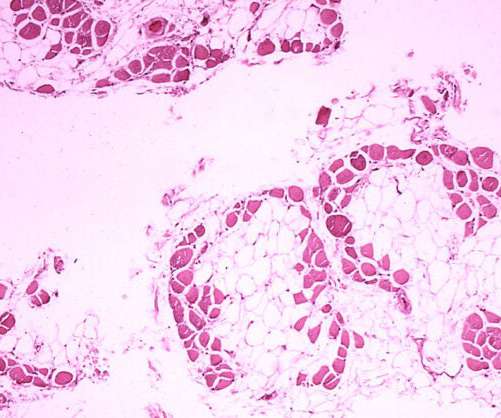
Pharmaceutical Technology
NOVEMBER 1, 2022
The US Food and Drug Administration (FDA) has granted Fast Track designation for Dyne Therapeutics’ DYNE-251 to treat Duchenne muscular dystrophy (DMD) mutations amenable to exon 51 skipping. The post FDA grants Fast Track status for Dyne’s Duchenne muscular dystrophy therapy appeared first on Pharmaceutical Technology.

The FDA Law Blog
APRIL 25, 2023
This provision became effective as of March 29, 2023. It will become part of the “refuse to accept” (RTA) checklist on October 1, 2023. The primary vehicle for FDA to request cybersecurity information in premarket submissions has been guidance documents. Timeline Section 524B became effective on March 29, 2023.

Pharmaceutical Technology
APRIL 21, 2023
On 14 April, the FDA rejected Lilly’s biologic licence application (BLA) for their anti-interleukin (IL)-23, mirikizumab, which is in development for the treatment of ulcerative colitis (UC). The recent setback dents mirikizumab’s chances to be the first among the IL-23 inhibitors to launch in the US for UC.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content