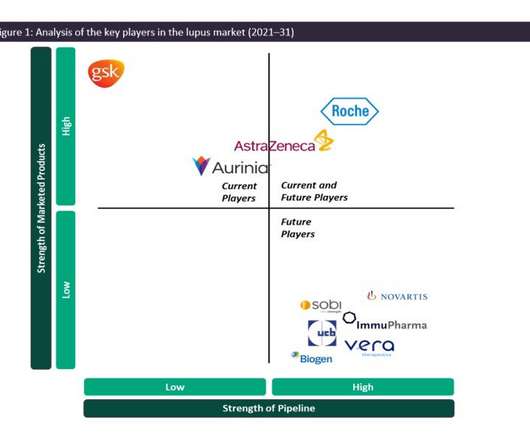
Current and future players in the lupus market
Pharmaceutical Technology
JANUARY 23, 2023
The management of SLE and LN consists largely of treatment options that are based on highly efficacious steroids or immune-suppressive therapy with an undesirable safety profile, which includes accrued organ damage, infections and cancer. in 2018 across the 7MM despite being an off-label therapy for lupus.




































Let's personalize your content