FDA-Approved Labeling: Is Enough Enough?
The FDA Law Blog
AUGUST 21, 2023
Livornese — I saw the sign…and the answer is no—FDA-approved labeling apparently is not enough under state failure-to-warn laws, according to certain courts.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

The FDA Law Blog
AUGUST 21, 2023
Livornese — I saw the sign…and the answer is no—FDA-approved labeling apparently is not enough under state failure-to-warn laws, according to certain courts.

The FDA Law Blog
DECEMBER 8, 2022
Food and Drug Administration (FDA) issued two guidance documents, one draft and one final, on food allergen labeling requirements. 1, 2023; The applicability of food allergen labeling requirements to specific products (e.g., 1, 2023; The applicability of food allergen labeling requirements to specific products (e.g.,
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

The FDA Law Blog
MAY 23, 2023
Food and Drug Administration (FDA) released a draft update to its Compliance Policy Guide (CPG) for FDA staff on the Agency’s enforcement of major food allergen labeling and cross-contact. Part 117, and the Food Allergy Safety, Treatment, Education and Research Act (2021). By Sophia R.

The Checkup by Singlecare
NOVEMBER 9, 2023
Food and Drug Administration (FDA) just approved Zepbound (tirzepatide) for chronic weight management. The injectable medication is a new version of Eli Lilly’s Mounjaro, which is approved by the FDA to control blood sugar in people with Type 2 diabetes. Zepbound, on the other hand, has been FDA-approved for weight loss.

pharmaphorum
SEPTEMBER 23, 2022
The FDA has granted accelerated approval the RET inhibitor for all adults with locally advanced or metastatic solid tumours with a RET fusion mutation, two years after giving it a green light for RET-positive thyroid and lung cancers. The post Lilly bags label extension for RET drug Retevmo in US appeared first on.

European Pharmaceutical Review
NOVEMBER 22, 2022
The US Food and Drug Administration ( FDA ) has accepted priority review of AbbVie’s Biologics License Application of epcoritamab (DuoBody®-CD3xCD20), an investigational subcutaneous bispecific antibody (BsAb), for adults with relapsed/refractory large B-cell lymphoma (LBCL) after two or more lines of systemic therapy.

STAT
OCTOBER 4, 2023
Lumakras received an accelerated — or conditional — approval in May 2021, making it the first KRAS drug on the market. But in reviewing the study, the FDA found potential data and protocol problems that could jeopardize the entire trial. Now, Amgen needs to prove it works.
pharmaphorum
JANUARY 12, 2021
Boehringer and Eli Lilly have moved closer to a heart failure indication for their SGLT2 inhibitor Jardiance, as the FDA starts a fast-track review of the drug in its first use beyond diabetes. Farxiga won FDA approval for adults with HFrEF in May 2020, which helped to drive its third-quarter sales up by a third to $525 million.

pharmaphorum
JANUARY 4, 2023
Retail pharmacies in the US will be able to dispense mifepristone-based therapies to end pregnancies after the FDA introduced changes to its regulatory framework for the products. The American College of Obstetricians and Gynaecologists (ACOG) said the FDA’s decision was a victory for women’s health.

pharmaphorum
JANUARY 26, 2022
Immunocore has secured a piece of biotech industry history, becoming the first company to get an FDA approval for a cancer therapeutic based on T cell receptor (TCR) technology. The post Immunocore claims first-ever FDA approval for TCR cancer therapy appeared first on.

Pharmaceutical Technology
MAY 19, 2023
The US Food and Drug Administration (FDA) has accepted Satsuma Pharmaceuticals’ 505(b)(2) new drug application (NDA) for STS101 for acute treatment of migraine, for review. The company’s NDA was supported mainly by the data obtained from the Phase I clinical trial completed in June 2021, and the Phase III ASCEND trial.

STAT
JANUARY 8, 2024
Cure Ventures , a Boston-based venture capital firm founded in 2021 by a trio of veteran biotech investors, has brought in two former executives from Sage Therapeutics to prepare them for investing in neuroscience startups. Continue to STAT+ to read the full story…

pharmaphorum
JULY 19, 2022
The FDA’s decision to clear the new indication for the topical JAK1/JAK2 inhibitor came after a priority review that was delayed by a request for additional data by the regulator, holding back a decision by three months. The post FDA clears Incyte’s Opzelura as first vitiligo therapy appeared first on.
pharmaphorum
DECEMBER 29, 2021
Sanofi and Regeneron have another challenger to their big-selling drug Dupixent for atopic dermatitis, now that the FDA has approved Leo Pharma’s rival antibody tralokinumab. billion in the first nine months of 2021, helped by additional approvals in severe asthma and chronic rhinosinusitis with nasal polyposis.

The FDA Law Blog
JANUARY 1, 2024
Maybe that’s also why FDA last week publicized the highest number of important Warning Letters of the year (compared with prior releases in 2023). Perhaps FDA wanted us to remember 2023 as the year FDA succeeded in uncovering critical defects in drug and device manufacturing, and in critical trials. Dextrum Laboratories Inc.
Pharmaceutical Technology
MAY 26, 2023
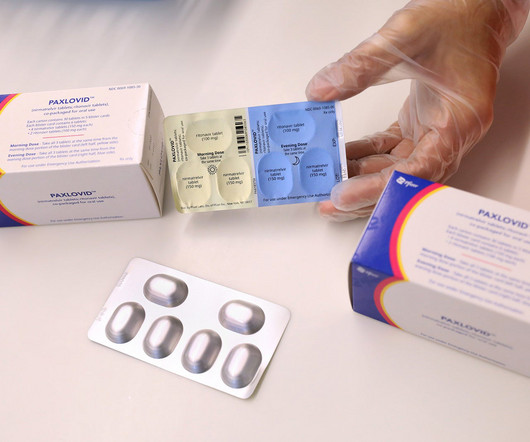
After earning multimillion dollar revenues while being an authorised preferred treatment for Covid-19, the US Food and Drug Administration (FDA) has granted a full approval to Pfizer’s oral antiviral Paxlovid (nirmatrelvir + ritonavir).

The FDA Law Blog
JULY 10, 2023
After a firm submits a 510(k) to FDA, FDA will request still more information after a first-pass review. According to the 2 nd Quarter FY2023 MDUFA V Performance Report , FDA issued a request for additional information (AI request) on the first FDA review cycle for 63% to 68% of 510(k)s submitted in FY2018 to FY2022.
pharmaphorum
MAY 26, 2022
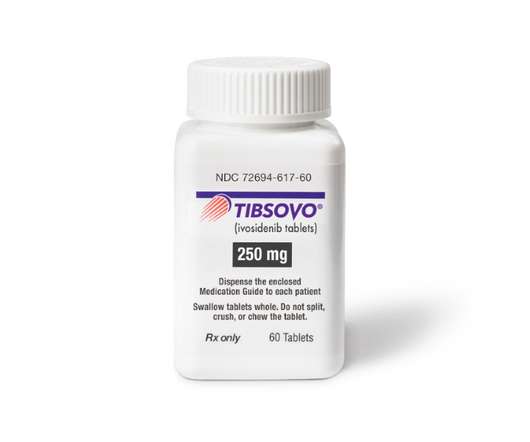
billion takeover of Agios Pharma’s oncology business after getting a key FDA approval for Tibsovo, the main asset in the deal. As Vidaza is a standard first-line therapy for AML the new indication makes Tibsovo an option for a wider range of patients, although the drug has reportedly been used off-label in this setting.

pharmaphorum
FEBRUARY 8, 2021
Bristol-Myers Squibb finally has FDA approval for its CAR-T therapy liso-cel, which has been cleared by the US regulator as Breyanzi for certain forms of large B-cell lymphoma. DLBCL is the most common type of NHL in adults, accounting for around a third of the 77,000 new cases diagnosed in the US, according to the FDA.

The FDA Law Blog
AUGUST 23, 2022
By doing so, FDA has limited the number of tests that have reached the market, thereby reducing available supply and increasing prices. As FDA would acknowledge, the antigen tests are the fastest and most practical method for distributing testing in the general population. That could happen again.

pharmaphorum
JUNE 8, 2021
The FDA “has failed in its responsibility to protect patients and families from unproven treatments with known harms” in approving Biogen’s Alzheimer’s disease drug Aduhelm. Patrizia Cavazzoni (@FDACDERDirector) June 7, 2021. Broad label questioned. — Brad Loncar (@bradloncar) June 7, 2021.

pharmaphorum
MARCH 1, 2022
The US Food and Drug Administration (FDA) has approved a new cell-based therapy for blood cancer, developed by Janssen and China’s Legend Biotech to treat multiple myeloma. Following the FDA decision, CARVYKTI is now Legend’s first product to be approved for use in the US.
pharmaphorum
SEPTEMBER 21, 2021
Antibody-drug conjugate (ADC) specialist Seagen has claimed its fourth product approval in the US, getting the nod from the FDA for Tivdak as a second-line monotherapy for recurrent or metastatic cervical cancer. There’s no word yet on a price for Tivdak, which will launch with a black box warning for ocular toxicity on its label.

pharmaphorum
DECEMBER 13, 2022
Amgen has its first direct competition in the KRAS inhibitor class following FDA approval of Mirati’s adagrasib as Krazati for a form of lung cancer. The FDA approval is conditional on a positive outcome from a phase 3 confirmatory trial – KRYSTAL-12 – which is scheduled to complete next year.
pharmaphorum
DECEMBER 16, 2020
An FDA advisory committee 12 to 1 in favour of approving Entresto (sacubitril/valsartan) for heart failure with preserved ejection fraction (HFpEF), which accounts for around half of all heart failure cases but proves highly resistant to drug treatment. The post Entresto set for big sales hike after FDA panel endorsement appeared first on.
pharmaphorum
JUNE 11, 2021
Sanofi’s hopes of a speedy approval of first-in-class C1s inhibitor sutimlimab in rare disorder cold agglutinin disease (CAD) were dashed by the FDA, but new phase 3 data keep the drugmaker on track for a resubmission before year-end. At the moment CAD is managed using off-label therapies, including rituximab and corticosteroid drugs.

pharmaphorum
MAY 27, 2021
Myovant has chalked up a second approval for its flagship drug relugolix from the FDA, becoming the first once-daily oral drug in the US to treat heavy menstrual bleeding associated with uterine fibroids. . The post FDA clears Myovant’s relugolix for uterine fibroids, setting up AbbVie clash appeared first on.

pharmaphorum
JULY 11, 2021
In another twist to the Aduhelm approval tale, FDA’s Janet Woodcock has called for an independent investigation into the relationship between agency staffers and Biogen executives. In the letter, she writes: “FDA’s decisions are far ranging and it is inevitable that some of those decisions will lead to controversy.”

The FDA Law Blog
NOVEMBER 29, 2023
Since that time, FDA issued a draft guidance for predetermined change control plans (PCCPs) for Artificial Intelligence/Machine Learning (AI/ML) software functions. FDA considers these guiding principles as complimentary to their recent efforts around PCCPs including their proposed draft guidance on PCCPs.

pharmaphorum
OCTOBER 27, 2022
The FDA has accepted for review Seres Therapeutics’ Biologics License Application (BLA) for SER-109, an investigational oral microbiome therapeutic for the prevention of recurrent Clostridium difficile infection (rCDI). If approved, SER-109 would be the first-ever FDA-approved oral microbiome therapeutic. Now at $6.79
The Checkup by Singlecare
MARCH 25, 2024
Wegovy (semaglutide) is an injectable medication approved in 2021 for people with obesity or who are overweight and have a weight-related medical condition. Because those drugs are not FDA approved for weight loss, like Wegovy, healthcare providers may prescribe them off-label.

The FDA Law Blog
APRIL 30, 2023
Senate Committee on Health, Education, Labor and Pensions (“Senate HELP”) is scheduled to take up legislation that could significantly limit access to the courts and immunize critical FDA decisions from timely judicial review. Subsection 505(q) initially was added by Section 914 of the 2007 FDA Amendments Act (“FDAAA”), Pub.

pharmaphorum
JUNE 7, 2021
Kymriah (tisagenlecleucel) was approved for relapsed/refractory ALL in 2017, but its label covers use in children and young adults aged up to 25, who account for the bulk of cases of the blood cancer. It was filed for the adult ALL indication in the US in April, and has a priority review from the FDA, with a verdict due in October.

The Checkup by Singlecare
JANUARY 26, 2024
Approved by the Food and Drug Administration (FDA) in 2017 for blood sugar management, Ozempic has recently gained attention for its weight loss effects. Healthcare professionals prescribe Ozempic off-label for weight management for patients with obesity. It received FDA approval for weight management in June 2021.)

STAT
JUNE 29, 2023
He allegedly worked with several other individuals and pharmacies between 2017 and 2021 as part of the scheme. Reconfiguring bottles and labels for distributing medicines is known as misbranding or diverting legitimate prescription drugs. Steven Diamantstein, who runs Scripts Wholesale in Brooklyn, N.Y.,

The Checkup by Singlecare
DECEMBER 1, 2023
Red Dye 40 is among the most commonly used artificial food dyes in the United States, and you might see it labeled under a different name on the products you consume. Regardless of what manufacturers label it as, there have been ties to specific negative side effects when ingested too frequently. 40 Aluminum Lake, and FD&C Red No.

The Checkup by Singlecare
FEBRUARY 8, 2024
Drug interactions | Food interactions | Other interactions | Avoiding interactions | When to see a doctor Mounjaro (tirzepatide) is gaining popularity as a treatment for Type 2 diabetes mellitus and off-label for weight management. Cases of diabetic retinopathy with tirzepatide use were noted in a 2021 study.

Pharmaceutical Technology
JULY 20, 2022
On 6 July, in an effort to accelerate access, the US Food and Drug Administration (FDA) allowed pharmacists to also begin prescribing the drug to eligible individuals with Covid-19. In its announcement, the FDA advised individuals to keep their electronic or printed health records from the past year ready while seeking treatment.
pharmaphorum
OCTOBER 1, 2020
Shares in US biotech CTI BioPharma have shot up after the FDA agreed to an accelerated review early next year of its lead drug pacritinib for low blood platelets (thrombocytopenia) caused by myelofibrosis. The post CTI wins over FDA to claim early review of myelofibrosis drug appeared first on. after the announcement.

The FDA Law Blog
OCTOBER 24, 2022
355g(a), directed FDA to “establish a program to evaluate the potential use of real world evidence” both “to help to support the approval of a new indication” and “to help to support or satisfy postapproval study requirements.” The sponsor and FDA reach agreement on the study design information to be publicly disclosed.

The FDA Law Blog
OCTOBER 24, 2023
Mullen — As of October 1, 2023, all 510(k) submissions, unless exempted, must be submitted to FDA using the electronic Submission Template And Resource ( eSTAR ). Currently, eSTAR is voluntary for medical device De Novo submissions, but FDA has initiated the process of requiring De Novos to be submitted using eSTAR. 860.230.

The FDA Law Blog
MAY 8, 2023
Javitt — FDA recently published a long-awaited draft guidance aimed at reducing the need for prior FDA authorization of modifications to artificial intelligence/machine learning (AI/ML)-enabled device software functions (ML-DSFs). Baumhardt, Senior Medical Device Regulation Expert & Philip Won & Gail H. See 21 CFR 807.81(a)(3)

The Checkup by Singlecare
DECEMBER 5, 2023
California Florida New York Texas Illinois New Jersey Colorado North Carolina Ohio Washington Additionally, a separate study by Trilliant found that cities in the states mentioned above had the highest percentage change in Ozempic use from 2021 to 2022. It should not be prescribed to patients who want to lose just a few pounds.

The FDA Law Blog
JUNE 22, 2023
Mullen — FDA has been clearing over-the-counter (OTC) in vitro diagnostic (IVD) tests nearly since the beginning of its premarket regulation of devices. The first OTC IVD cleared by FDA was a qualitative dipstick urine glucose test in 1977, followed shortly thereafter by the first OTC pregnancy test clearance in 1978. lay users).
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content