FDA-Approved Labeling: Is Enough Enough?
The FDA Law Blog
AUGUST 21, 2023
Livornese — I saw the sign…and the answer is no—FDA-approved labeling apparently is not enough under state failure-to-warn laws, according to certain courts.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

The FDA Law Blog
AUGUST 21, 2023
Livornese — I saw the sign…and the answer is no—FDA-approved labeling apparently is not enough under state failure-to-warn laws, according to certain courts.

STAT
AUGUST 21, 2023
A 2019 policy requires companies that make unhealthy foods to include warning labels on the front of any boxes they sell in Mexico to educate consumers about things like excess sugar and fat. MEXICO CITY — Kellogg’s is waging a war here over Tigre Toño and Sam el Tucán.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

The FDA Law Blog
DECEMBER 15, 2022
By Riëtte van Laack — On December 7, 2022, FDA announced the availability of the final guidance regarding the enforcement policy for homeopathic drug products. This concludes FDA’s reevaluation of the regulation of homeopathic drugs which it started in 2015. It issued a draft guidance in 2017 which was subsequently revised in 2019.

pharmaphorum
OCTOBER 14, 2020
Wakix (pitolisant) was first approved in August 2019 for treatment of excessive sleepiness in adults with narcolepsy. It is the only FDA-approved drug to treat cataplexy associated with narcolepsy not scheduled as a controlled substance by the US Drug Enforcement Administration. Last year, the FDA rejected Wakix for the additional use.

pharmaphorum
SEPTEMBER 23, 2022
The FDA has granted accelerated approval the RET inhibitor for all adults with locally advanced or metastatic solid tumours with a RET fusion mutation, two years after giving it a green light for RET-positive thyroid and lung cancers. Lilly acquired Retevmo as part of its $8 billion takeover of Loxo Oncology in 2019.

pharmaphorum
DECEMBER 22, 2021
Amgen has won FDA approval for a stronger label for its oral plaque psoriasis therapy Otezla, as it prepares for competition from Bristol-Myers Squibb’s much-touted rival deucravacitinib, which could make its debut next year. The post Amgen builds Otezla’s psoriasis label as rival BMS looms large appeared first on.

Pharmaceutical Technology
AUGUST 8, 2022
AstraZeneca and Daiichi Sankyo ’s Enhertu (trastuzumab deruxtecan) has received expanded approval from the US Food and Drug Administration (FDA) to treat adults with unresectable or metastatic HER2-low (IHC 1+ or IHC 2+/ISH-) breast cancer.

pharmaphorum
AUGUST 21, 2022
Shares in Axsome Therapeutics have rocketed on FDA approval of its depression therapy Auvelity (formerly AXS-05) – a year after its approval was held up by the regulator. That status goes to Johnson & Johnson’s Spravato (esketamine), which was approved in March 2019 and is administered as a nasal spray twice a week for four weeks.
pharmaphorum
JANUARY 12, 2021
Boehringer and Eli Lilly have moved closer to a heart failure indication for their SGLT2 inhibitor Jardiance, as the FDA starts a fast-track review of the drug in its first use beyond diabetes. Farxiga won FDA approval for adults with HFrEF in May 2020, which helped to drive its third-quarter sales up by a third to $525 million.
pharmaphorum
JULY 20, 2021
The FDA has taken a longer than usual look at Ardelyx’ regulatory submission for tenapanor, a drug for high blood phosphate levels associated with chronic kidney disease (CKD), and found it wanting – sending the biotech’s shares into a tailspin. “Despite….advantageous “Despite….advantageous

pharmaphorum
AUGUST 18, 2021
The FDA has approved GlaxoSmithKline’s latecomer PD-1 inhibitor Jemperli for a second use that will significantly extend the patient population eligible for treatment with the drug. billion takeover of Tesaro in 2019. The post GSK claims broader ’tissue-agnostic’ label for PD-1 drug Jemperli appeared first on.

pharmaphorum
JANUARY 4, 2023
Retail pharmacies in the US will be able to dispense mifepristone-based therapies to end pregnancies after the FDA introduced changes to its regulatory framework for the products. The American College of Obstetricians and Gynaecologists (ACOG) said the FDA’s decision was a victory for women’s health.

pharmaphorum
JULY 26, 2021
Fresh from its takeover of Alexion, AstraZeneca has picked up a recommendation in the EU for an expansion of the label of Ultomiris, one of the main assets behind the $39 billion merger. Ultomiris was approved by the FDA for use in paediatric PNH last month.

pharmaphorum
SEPTEMBER 2, 2021
The FDA has concluded its safety review of Pfizer’s JAK inhibitor Xeljanz and Xeljanz XR, requiring revised warnings for the drugs as well as others in the class after finding evidence of elevated risks of serious heart-related events. The post FDA firms up JAK inhibitor warnings after Xeljanz review appeared first on.

pharmaphorum
AUGUST 4, 2021
The HDAC inhibitor was given an accelerated approval by the FDA as a second-line PTCL therapy on the strength of overall response data, but a phase 3 study comparing Istodax to first-line chemotherapy showed no improvement on progression-free survival (PFS).

pharmaphorum
JANUARY 10, 2022
Swiss biotech Idorsia has claimed FDA approval for its first product – insomnia treatment Quviviq – setting up a market challenge to class rivals from Merck & Co and Eisai. Belsomra’s first-to-market advantage hasn’t translated into big annual sales, which have stayed planted just over the $300 million mark since 2019.
pharmaphorum
SEPTEMBER 20, 2021
Samsung Bioepis and Biogen have claimed the first FDA approval for a biosimilar version of Roche and Novartis’ Lucentis (ranibizumab) for leading causes of blindness, raising the prospect of a cheaper treatment option for US patients. Sales reached a peak in 2019, when Roche booked $1.8 billion, but fell back to $1.4

The FDA Law Blog
JULY 10, 2023
After a firm submits a 510(k) to FDA, FDA will request still more information after a first-pass review. According to the 2 nd Quarter FY2023 MDUFA V Performance Report , FDA issued a request for additional information (AI request) on the first FDA review cycle for 63% to 68% of 510(k)s submitted in FY2018 to FY2022.

The Checkup by Singlecare
MARCH 12, 2024
If you see medications labeled “Advil” and others labeled “ibuprofen,” you may ask yourself: “Is Advil the same as ibuprofen?” NSAIDs are FDA-approved pain medications that can decrease fever and inflammation. You may also want to know: Does Advil have ibuprofen in it? What does Advil and ibuprofen treat?

The FDA Law Blog
JUNE 11, 2023
However, one piece, excerpted below, stood out to us: Historically, FDA has at times issued WRs solely for studies required under PREA, even if there were no other indications that may produce health benefits in the pediatric population. However, over time, data on pediatric labeling changes pursuant to BPCA and/or PREA have been collected.

pharmaphorum
AUGUST 16, 2021
Merck & Co has extended its range of cancer medicines in the US after claiming FDA approval for Welireg – a drug it acquired as part of its $2.2 billion takeover of Peloton Therapeutics in 2019 – for a family of rare tumours. Merck paid $1.05 billion upfront to secure rights to Welireg, and also pledged another $1.15
The Checkup by Singlecare
DECEMBER 21, 2023
But since its FDA approval for the treatment of Type 2 diabetes back in 1994, metformin has emerged as something of a “miracle drug” thanks to its effectiveness in treating other common health conditions, like infertility, obesity , and heart disease. Metformin is a medication commonly used to treat people with Type 2 diabetes.

pharmaphorum
MAY 25, 2022
Roivant group company Dermavant has made the transition to a commercial-stage company after getting FDA approval for Vtama, a drug for psoriasis that it claims could be a game-changer in the treatment of the skin disorder. The post Dermavant claims its first approval as FDA clears psoriasis drug appeared first on.

pharmaphorum
MAY 27, 2021
Myovant has chalked up a second approval for its flagship drug relugolix from the FDA, becoming the first once-daily oral drug in the US to treat heavy menstrual bleeding associated with uterine fibroids. . The post FDA clears Myovant’s relugolix for uterine fibroids, setting up AbbVie clash appeared first on.
pharmaphorum
FEBRUARY 5, 2021
The FDA has said it will wait for additional results from last week’s trial showing an increased risk of cardiac side effects with Pfizer’s arthritis blockbuster Xeljanz before deciding on further action. The post FDA holds back from action over Xeljanz safety study appeared first on.

pharmaphorum
FEBRUARY 23, 2021
The FDA is to begin a fast review of Incyte’s Jakafi (ruxolitinib) for patients with chronic graft-versus-host disease (GVHD), which cannot be treated with steroids. The post FDA to quickly review Incyte’s Jakafi in chronic GVHD appeared first on.

The Checkup by Singlecare
DECEMBER 1, 2023
Red Dye 40 is among the most commonly used artificial food dyes in the United States, and you might see it labeled under a different name on the products you consume. Regardless of what manufacturers label it as, there have been ties to specific negative side effects when ingested too frequently. 40 Aluminum Lake, and FD&C Red No.

The FDA Law Blog
FEBRUARY 16, 2023
Richardson — On February 15, 2023, the Nonprescription Drugs Advisory Committee (NDAC) and the Anesthetic and Analgesic Drug Products Advisory Committee (AADPAC) held a joint meeting to discuss an application pending before FDA that would switch Narcan (naloxone) Nasal Spray from prescription to over-the-counter (OTC) status.

PharmaShots
FEBRUARY 6, 2023
Shots: The company reported the results from the PoC P-II open-label (UNITY) trial evaluating nipocalimab (IV, qw) in 14 patients who are at high risk for sev. HDFN The trial met its 1EPs i.e., the results showed that patients treated with nipocalimab achieved a live birth at or after the gestational age of 32wks.

pharmaphorum
MARCH 1, 2021
European regulators have refused to back GlaxoSmithKline’s daily triple therapy Trelegy Ellipta for asthma, denying a label extension because there was no evidence to show a reduction of flare-ups. GSK’s problems with Trelegy, developed in partnership with Innoviva, stem from the phase 3 CAPTAIN study , which reported findings in May 2019.

pharmaphorum
NOVEMBER 30, 2021
The FDA has kicked off its review of Bristol-Myers Squibb’s psoriasis therapy deucravacitinib, setting an action date of 1 September next year, as the EU and Japanese regulators also start their appraisals of the drug. The post FDA sets Sept review date for BMS’ psoriasis drug deucravacitinib appeared first on.
pharmaphorum
SEPTEMBER 11, 2022
Bristol-Myers Squibb’s deucravacitinib– one of the main pipeline assets in its $74 billion takeover of Celgene in 2019 – has been approved in its first market as a treatment for moderate-to-severe plaque psoriasis. billion oral psoriasis therapy Otezla (apremilast). Otezla was acquired by Amgen for $13.4

The FDA Law Blog
MAY 8, 2023
Javitt — FDA recently published a long-awaited draft guidance aimed at reducing the need for prior FDA authorization of modifications to artificial intelligence/machine learning (AI/ML)-enabled device software functions (ML-DSFs). Baumhardt, Senior Medical Device Regulation Expert & Philip Won & Gail H. See 21 CFR 807.81(a)(3)

The Checkup by Singlecare
FEBRUARY 5, 2024
Food and Drug Administration (FDA) for relief of mild, intermittent asthma symptoms, like wheezing, cough, shortness of breath, and chest tightness. It is approved and labeled for use in patients 12 and older who have been previously diagnosed with asthma. OTC inhalers are approved by the U.S.

The Checkup by Singlecare
DECEMBER 28, 2023
Although Ozempic has been approved by the Food and Drug Administration (FDA) since 2017, its status as a household name is relatively recent. Weight loss is an off-label , non-FDA-approved use for Ozempic. The FDA has urged consumers to use caution when taking medication from these compound pharmacies.
pharmaphorum
JULY 9, 2021
According to the EMA’s pharmacovigilance risk assessment committee (PRAC), labelling for Pfizer/BioNTech’s Comirnaty and Moderna’s Spikevax should be updated to reflect “very rare cases” of myocarditis and pericarditis with the shots. . The therapy hasn’t yet been approved by the FDA.
The People's Pharmacy
MARCH 20, 2023
Over the past several years, the FDA has approved a number of injectable medications designed to help people with type 2 diabetes control their blood sugar. Like liraglutide, this injectable medication acts like glucagon in the body ( Frontiers in Endocrinology , April 12, 2019 ). Another Question About Ozempic: Q.

pharmaphorum
JUNE 6, 2022
The results have set up a pre-filing meeting with the FDA, according to BMS. Rozlytrek was approved by the FDA in 2019 but has just started to gather sales momentum, making around $17 million in the first quarter of this year, an increase of 78% on the first three months of 2021.
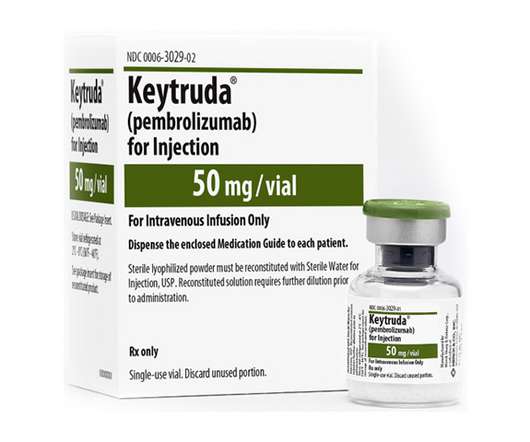
pharmaphorum
OCTOBER 25, 2021
The new approval is the first breast cancer indication for Keytruda in Europe, although elsewhere Merck has already started to expand the label for its cancer immunotherapy in TNBC. The US and Japanese regulators approved the Keytruda plus chemo regimen for first-line TNBC treatment earlier this year.
The Checkup by Singlecare
AUGUST 29, 2023
Food and Drug Administration (FDA) in 2012 as a brand-name drug. Although a generic was approved in 2019, generic apixaban will not be available on the market until at least April 2028. The first generics of apixaban were announced in December 2019. This is called off-label prescribing. Eliquis was approved by the U.S.
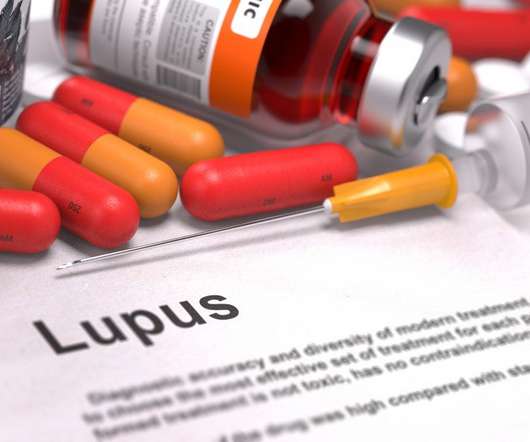
Pharmaceutical Technology
DECEMBER 5, 2022
Notably, Lupkynis is the first oral therapy approved by the US Food and Drug Administration (FDA) and EC for the treatment of active LN. Overall, Aurinia’s Lupkynis is expected to face tough competition from Benlysta in LN, which received FDA approval in December 2020 and EMA approval in May 2021.
The Checkup by Singlecare
OCTOBER 12, 2023
Abilify generic | Abilify vs. aripiprazole | Cost | Off-label use | How to switch Abilify is a brand-name medication approved to treat certain mental health conditions, including the treatment for schizophrenia , bipolar disorder , and major depressive disorder. Abilify first received FDA approval in 2002. mL, 960 mg/3.2
The Checkup by Singlecare
APRIL 25, 2024
Labeled as a controlled substance , Adderall contains amphetamine and dextroamphetamine. Food and Drug Administration ( FDA ). The FDA explains that their regulations require a proposed expiration date for medications to be approved. Pharmaceuticals have expiration dates. Adderall falls into this category.

pharmaphorum
NOVEMBER 10, 2022
Sales were around $140 million in 2019, and climbed to $165 million the following year, despite the impact of the pandemic. With finances tight, Clovis has deferred a $1.9 million interest payment on debt, and is now in a 30-day grace period that could lead to it being in default.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content