Methodology to Define a Pharma 4.0™ Roadmap
ISPE
MAY 11, 2023
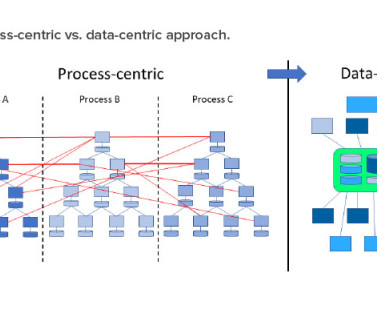
The key to data integrity compliance is a well-functioning data governance system 1 , 2 in which the data flow path for all business processes and equipment—such as in manufacturing, laboratory, and clinical studies—is fully understood and documented by a detailed process data flow map. initiatives. to Industry 4.0







Let's personalize your content