Is a lack of real-time data holding trials back?
pharmaphorum
SEPTEMBER 11, 2020
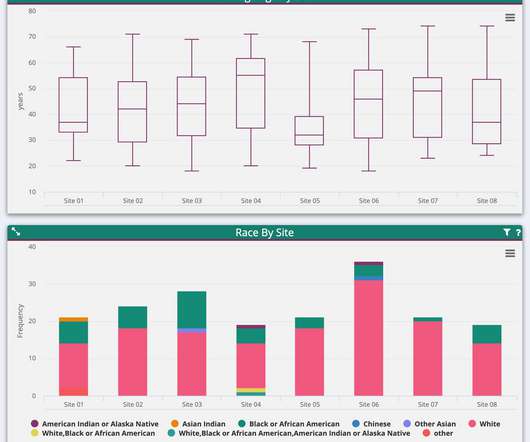
Their findings, published in the September 2018 research report Challenges and Opportunities in Clinical Data Management, underscore the severity of the problem. Delayed data delivery. Study data is typically collected by internal and external partners. Potentially catastrophic setbacks. That’s transformative.








Let's personalize your content