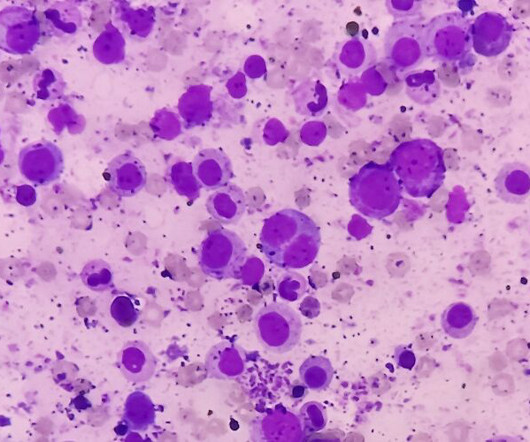
The changing treatment landscape of acute lymphoblastic leukaemia
Hospital Pharmacy Europe
AUGUST 29, 2024
The CAR T-cell therapy tisagenlecleucel has recently been given the green light for routine rollout on the NHS to treat paediatric patients with acute lymphoblastic leukaemia. These patients often reach the ceiling for toxicity in terms of chemotherapy and radiotherapy in the context of a bone marrow transplant.










Let's personalize your content