Chemotherapy prescribing habits for stage 3 colon cancer changed following IDEA study
Hospital Pharmacy Europe
AUGUST 14, 2023
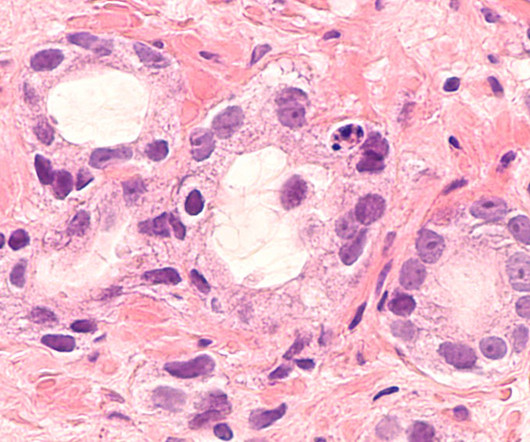
A significant and increasing trend towards the use of a three-month rather than six-month adjuvant chemotherapy regime in stage 3 colon cancer has been observed following publications from the IDEA collaboration abstract. But whether treatments regimens could be shorter while maintaining efficacy remained uncertain.




































Let's personalize your content