Orchard’s MLD gene therapy becomes costliest US medicine
pharmaphorum
MARCH 20, 2024
Orchard Therapeutics has revealed the US price of Lenmeldy, its gene therapy for rare disease MLD, placing a $4.25m price tag on the one-shot treatment
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

 tag therapeutics
tag therapeutics 
pharmaphorum
MARCH 20, 2024
Orchard Therapeutics has revealed the US price of Lenmeldy, its gene therapy for rare disease MLD, placing a $4.25m price tag on the one-shot treatment

Pharmaceutical Technology
OCTOBER 17, 2023
The Netherlands-based company’s treatment has been awarded the designation by the US FDA following a successful Phase I trial.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Fierce Pharma
NOVEMBER 7, 2023
Vertex and CRISPR Therapeutics are up first with a Dec. | But on Tuesday bluebird said that Vertex’s price tag will not factor into how the Massachusetts company will price its treatment. Two long-awaited treatments for sickle cell disease (SCD) are on the docket for FDA decisions next month.

PharmaShots
MARCH 24, 2023
PTC Therapeutics’ Upstaza (eladocagene exuparvovec) Receives NICE Recommendation for the Treatment of AADC Deficiency Date: Mar 24, 2023 | Tags: PTC Therapeutics, Upstaza, eladocagene exuparvovec, AADC Deficiency, Regulatory, NICE Onconova Therapeutics Entered into a Research Collaboration with Pangea Biomed to Identify Biomarkers for Cancer (..)
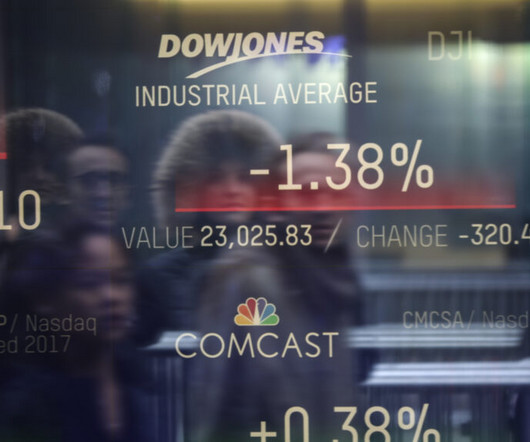
STAT
OCTOBER 5, 2023
LONDON — The Japanese firm Kyowa Kirin is acquiring gene therapy maker Orchard Therapeutics in a $400 million deal, the companies said Thursday. Kyowa Kirin is paying $16 per share — a nearly 100% premium on Orchard’s Wednesday closing price — in cash, amounting to roughly $390 million.

PharmaShots
APRIL 7, 2023
TG Therapeutics Receives EMA’s CHMP Positive Opinion of Briumvi (ublituximab-xiiy) for the Treatment of Relapsing Forms of Multiple Sclerosis Date: Apr 3, 2023 | Tags: TG Therapeutics, Briumvi, Ublituximab-xiiy, Multiple Sclerosis, Regulatory, EMA, CHMP BMS Receives EMA’s CHMP Positive Opinion of Breyanzi (lisocabtagene maraleucel) for (..)

PharmaShots
JUNE 2, 2023
Impact Therapeutics Entered into a License and Collaboration Agreement with Eikon Therapeutics to Develop and Commercialize IMP1734 Date: June 02, 2023 | Tags: Impact Therapeutics, Eikon Therapeutics, IMP1734, Pharma, China, Hong Kong, Macau, Taiwan BMS’ Mavacamten Receives the NICE Recommendation for the Treatment of Obstructive Hypertrophic (..)

PharmaShots
MAY 12, 2023
Sanofi Reports P-IIIb Trial (HARMONIE) Results of Nirsevimab for the Prevention of Hospitalizations due to RSV-Related LRTD Date: May 12, 2023 | Tags: Sanofi, Nirsevimab, RSV-Related LRTD, Clinical Trial, P-IIIb, HARMONIE Trial G1 Therapeutics Presents Preliminary Results from P-II Trial of Trilaciclib for Triple-Negative Breast Cancer at ESMO 2023 (..)

PharmaShots
MAY 5, 2023
Chiesi Global Rare Diseases and Protalix BioTherapeutics Receive EC’s Marketing Authorization of PRX-102 (pegunigalsidase alfa) for the Treatment of Fabry Disease Date: May 05, 2023 | Tags: Chiesi Global Rare Diseases, Protalix BioTherapeutics, PRX-102, pegunigalsidase alfa, Fabry Disease, Regulatory, EC, Marketing Authorization Kinoxis Therapeutics (..)

PharmaShots
JUNE 23, 2023
Gilead Receives EMA’s CHMP Positive Opinion of Trodelvy (sacituzumab govitecan) for Pre-Treated HR+/HER2- Metastatic Breast Cancer Date: June 23, 2023 | Tags: Gilead, Trodelvy, sacituzumab govitecan, HR+/HER2- Metastatic Breast Cancer, Regulatory, EMA, CHMP, Positive Opinion Sarepta Therapeutics’ Elevidys Receives the US FDA’s Accelerated (..)

PharmaShots
APRIL 28, 2023
Difficile Infection Date: Apr 27, 2023 | Tags: Seres Therapeutics, Nestlé Health Science, Vowst, C.

PharmaShots
JUNE 16, 2023
Date: June 12, 2023 | Tags: Novartis, Chinook Therapeutics, Atrasentan, Zigakibart, BION-1301, IgA nephropathy, M&A, ~$3.5B Date: June 12, 2023 | Tags: Novartis, Chinook Therapeutics, Atrasentan, Zigakibart, BION-1301, IgA nephropathy, M&A, ~$3.5B

PharmaShots
APRIL 14, 2023
Ocugen Reports Preliminary Results from the P-I/II Trial of OCU400 for the Treatment of Retinitis Pigmentosa and Leber Congenital Amaurosis Date: Apr 14, 2023 | Tags: Ocugen, OCU400, Retinitis Pigmentosa, Leber Congenital Amaurosis, Clinical Trial, P-I/II Trial Candesant Biomedical Receives the US FDA Clearance of Brella SweatControl Patch for Primary (..)

PharmaShots
JUNE 9, 2023
Quell Therapeutics Signed an Exclusive Option and License Agreement with AstraZeneca to Develop, Manufacture and Commercialize Engineered Treg Cell Therapies Date: June 09, 2023 | Tags: Quell Therapeutics, AstraZeneca, Engineered Treg Cell Therapies, Type 1 Diabetes, Inflammatory Bowel Disease, Biotech, Treg cell engineering modules Astellas and the (..)

PharmaShots
MARCH 31, 2023
PDS Biotech to Initiate P-III Trial (VERSATILE-003) of PDS0101 + Keytruda (pembrolizumab) for Head and Neck Cancer Date: Mar 31, 2023 | Tags: PDS Biotech, PDS0101, Keytruda, pembrolizumab, Head, Neck Cancer, Clinical Trial, P-III, VERSATILE-003 Trial Omega Therapeutics Entered into a Clinical Supply Agreement with Roche to Evaluate OTX-2002 for Hepatocellular (..)

PharmaShots
MARCH 24, 2023
PTC Therapeutics’ Upstaza (eladocagene exuparvovec) Receives NICE Recommendation for the Treatment of AADC Deficiency Date: Mar 24, 2023 | Tags: PTC Therapeutics, Upstaza, eladocagene exuparvovec, AADC Deficiency, Regulatory, NICE Onconova Therapeutics Entered into a Research Collaboration with Pangea Biomed to Identify Biomarkers for Cancer (..)
STAT
DECEMBER 12, 2023
The news comes as researchers are still processing the Food and Drug Administration’s landmark approval of two cutting-edge sickle cell therapies, one made by Vertex Pharmaceuticals and CRISPR Therapeutics and the other by Bluebird Bio. These drugs have price tags of $2.2 million and $3.1 million, respectively.

PharmaShots
FEBRUARY 3, 2023
4D Molecular Therapeutics Receives the US FDA’s IND Clearance of 4D-150 for the Treatment of Diabetic Macular Edema Date: Feb 03, 2023 | Tags: 4D Molecular Therapeutics, 4D-150, Diabetic Macular Edema, Regulatory, US, FDA, IND AstraZeneca and Amgen Receive the US FDA’s Approval of Tezspire (tezepelumab) for the Treatment of Severe Asthma (..)

PharmaShots
APRIL 21, 2023
Oblato Reports the First Patient Enrolment of OKN-007 in the P-I Clinical Trial for Recurrent High-Grade Glioma Date: Apr 21, 2023 | Tags: Oblato, OKN-007, Recurrent High-Grade Glioma, Regulatory, Henry Ford Health System CARsgen's CT041 Receives the US FDA’s IND Clearance for the Postoperative Adjuvant Therapy of Pancreatic Cancer Date: (..)

PharmaShots
JANUARY 27, 2023
Magenta Therapeutics Pauses the P-I/II Study in AML Patients Date: Jan 27, 2023 | Tags: Magenta Therapeutics, MGTA-117, AML, Clinical Trial, P-I/II Ipsen Receives CHMP Negative Opinion for Palovarotene to Treat Fibrodysplasia Ossificans Progressiva Date: Jan 27, 2023 | Tags: Ipsen, Palovarotene, Fibrodysplasia Ossificans Progressiva, Regulatory, CHMP, (..)

PharmaShots
FEBRUARY 24, 2023
Positive Solid Tumors Date: Feb 23, 2023 | Tags: Keymed, Lepu Biopharma, AstraZeneca, CMG901, Claudin 18.2-Positive Positive Solid Tumors Date: Feb 23, 2023 | Tags: Keymed, Lepu Biopharma, AstraZeneca, CMG901, Claudin 18.2-Positive Positive Solid Tumors Date: Feb 23, 2023 | Tags: Keymed, Lepu Biopharma, AstraZeneca, CMG901, Claudin 18.2-Positive

PharmaShots
FEBRUARY 17, 2023
Iveric Bio Reports the US FDA Acceptance of NDA and Granted Priority Review of Avacincaptad Pegol for Geographic Atrophy Date: Feb 17, 2023 | Tags: Iveric Bio, Avacincaptad Pegol, Geographic Atrophy, Regulatory, US, FDA, NDA, Priority Review ALX Oncology Reports the First Patient Dosing of Evorpacept in the P-I Study (ASPEN-07) for the Treatment of (..)

PharmaShots
MARCH 17, 2023
5-Adapted Bivalent Booster to Treat COVID-19 in Children ≤5 Years Date: Mar 15, 2023 | Tags: Pfizer, BioNTech, Omicron BA.4/BA.5-Adapted 5-Adapted Bivalent Booster to Treat COVID-19 in Children ≤5 Years Date: Mar 15, 2023 | Tags: Pfizer, BioNTech, Omicron BA.4/BA.5-Adapted

PharmaShots
FEBRUARY 10, 2023
LEO Pharma Reports P-III Trial (DELTA 2) Results of Delgocitinib for Chronic Hand Eczema Date: Feb 10, 2023 | Tags: LEO Pharma, Delgocitinib, Chronic Hand Eczema, Clinical Trial, P-III, DELTA 2 Trial GSK’s Jemperli (dostarlimab-gxly) Receives the US FDA’s Regular Approval for Recurrent or Advanced Mismatch Repair-Deficient Endometrial Cancer (..)

PharmaShots
FEBRUARY 10, 2023
LEO Pharma Reports P-III Trial (DELTA 2) Results of Delgocitinib for Chronic Hand Eczema Date: Feb 10, 2023 | Tags: LEO Pharma, Delgocitinib, Chronic Hand Eczema, Clinical Trial, P-III, DELTA 2 Trial GSK’s Jemperli (dostarlimab-gxly) Receives the US FDA’s Regular Approval for Recurrent or Advanced Mismatch Repair-Deficient Endometrial Cancer (..)

Pharmaceutical Technology
SEPTEMBER 30, 2022
Spark Therapeutics’ Luxturna, indicated for inherited retinal disease (IRD), was the first gene therapy to be approved, in 2017, with a price tag of $850,000 for each eye. Gene therapies for rare inherited diseases are very expensive, as they tend to be the only therapeutic option available to patients and are often curative.

PharmaShots
MAY 26, 2023
Takeda and HUTCHMED's Fruquintinib Receives Priority Review from the US FDA to Treat Metastatic Colorectal Cancer Date: May 26, 2023 | Tags: Takeda, HUTCHMED, Fruquintinib, Metastatic Colorectal Cancer, Regulatory, Priority Review, US, FDA Gilead Receives EMA’s CHMP Positive Opinion to Extend the Use of Veklury (remdesivir) for COVID-19 Date: (..)

PharmaShots
MAY 19, 2023
Alimera Acquires Rights from EyePoint Pharmaceuticals to Commercialise Yutiq in the US Date: May 19, 2023 | Tags: Alimera, EyePoint Pharmaceuticals, Yutiq, Iluvien, chronic non-infectious uveitis, US, Pharma Sobi Reports EMA’s Validation of MAA for Efanesoctocog Alfa to Treat Haemophilia A Date: May 19, 2023 | Tags: Sobi, Efanesoctocog Alfa, (..)
European Pharmaceutical Review
DECEMBER 9, 2022
billion by 2027 during the forecast period, due to the rise in ongoing clinical trials for oligonucleotide-based therapies in key therapeutic sectors such as oncology. Tag Copenhagen A/S (Denmark). Sarepta Therapeutics, Inc. (US). The research projected a compound annual growth rate (CAGR) of 16.8 percent from $7.7

pharmaphorum
APRIL 26, 2021
Technology firm Jolly Good and Teijin Pharma have begun a partnership to develop virtual reality digital therapeutics (VR DTx) for major depressive disorder. However Nokia famously ran into trouble when it tried to develop VR products for digital health and its OZO camera failed to catch on, mainly because of a high $60,000 price tag.

European Pharmaceutical Review
MAY 22, 2023
The review stated that therapeutic innovation has resulted in the production of expensive drugs to treat rare diseases, which need to be available on time. Smart contracts, which are essential for easing international trade and logistics operations, can enable the user to track fake returns to the producer and supplier using RFID tags.

pharmaphorum
JANUARY 11, 2022
A prescription digital therapeutic (DTx) for leukaemia patients developed by Blue Note Therapeutics has been awarded breakthrough device status by the FDA. The post Blue Note leukaemia DTx gets FDA breakthrough tag appeared first on. Other DTxs are aimed at people being treated for breast and lung cancers.
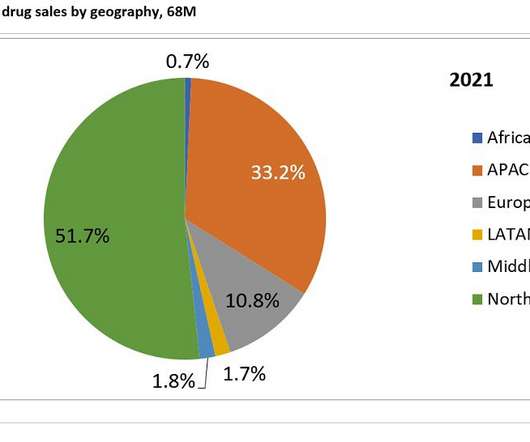
Pharmaceutical Technology
DECEMBER 15, 2022
Prior to the development of Camzyos and aficamten, obstructive HCM has not been the target of clinical trial development, so these two therapeutics would benefit a neglected patient population. of sales in 2021 and 2031 respectively. The second highest contributing market is APAC, generating 33.2%

pharmaphorum
JANUARY 18, 2022
The use of monovalent degraders, which enable the targeted degradation of disease-relevant proteins, offers a new avenue to reach tissue that cannot be targeted with conventional therapeutic agents. Researchers at Almirall have identified several proteins whose abnormal function is associated with inflammatory immune skin diseases.
pharmaphorum
NOVEMBER 24, 2022
One issue has been the cost of the drug, with health technology assessment agency NICE in the UK and the ICER organisation in the US both concluding its price tag means it is not a cost-effective option for health systems. The post J&J builds case for antidepressant Spravato with head-to-head trial appeared first on.
Pharmaceutical Technology
MAY 2, 2023
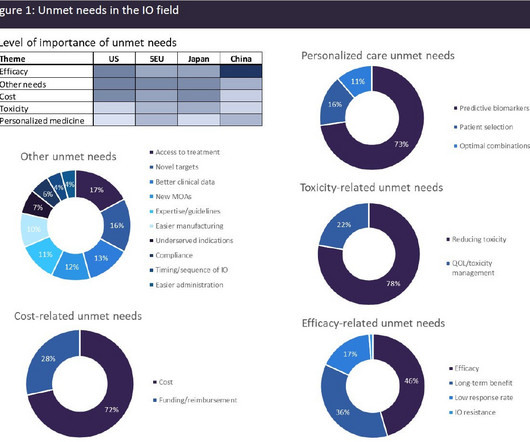
Immuno-oncology (IO) agents have transformed the cancer therapeutics landscape, driving long-term remissions in a subset of patients who historically had limited options. IO agents include the classes of immune checkpoint modulators, cell therapies, bispecific antibodies, oncolytic viruses, therapeutic vaccines, and cytokines.

pharmaphorum
NOVEMBER 24, 2021
Digital health company Pear Therapeutics has won FDA breakthrough device status for reSET-A, its development-stage prescription digital therapeutic (DTx) for people with alcohol-use disorder. The post Pear claims breakthrough tag for alcohol use disorder DTx appeared first on. Photo by thom masat on Unsplash.
Pharmaceutical Technology
DECEMBER 5, 2022
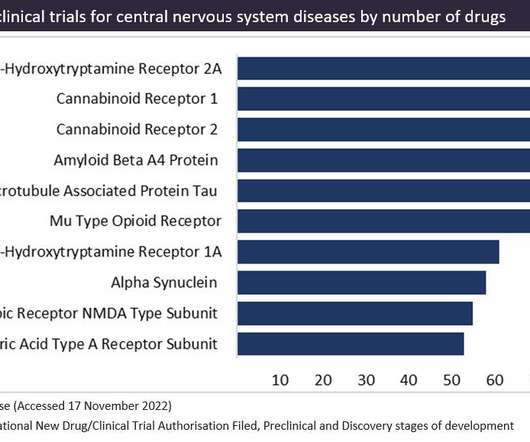
Cannabinoid receptors are a popular therapeutic target for cannabinoid-based drugs in the treatment of pain, neurological disorders and inflammation, according to GlobalData’s Pharma Intelligence Centre Drugs database. This is closely followed by CB2 receptors in second place.
pharmaphorum
OCTOBER 21, 2021
Vounatsos also insisted that Aduhelm’s price tag has not been a factor discouraging treatment with the drug. The slow roll out of Aduhelm raises pressure on Biogen to get the most out of Vumerity and other drugs like Sage Therapeutics-partnered antidepressant zuranolone, which is due to be filed next year.

pharmaphorum
AUGUST 21, 2022
Shares in Axsome Therapeutics have rocketed on FDA approval of its depression therapy Auvelity (formerly AXS-05) – a year after its approval was held up by the regulator. Auvelity is the first drug Axsome has taken through to regulatory approval, but could be the first of a string of new products for the company, according to Tabuteau.

European Pharmaceutical Review
AUGUST 24, 2022
ANTIBODY-DRUG conjugates (ADCs) are therapeutic molecules designed as highly targeted medicines with the promise of changing the way we treat cancer and other diseases. A key aspect in removing many existing hurdles lies in the linker and conjugation of the two therapeutic modalities while maintaining the properties of the naked antibody.
pharmaphorum
JUNE 12, 2022
Beti-cel has already been approved for marketing in Europe as Zynteglo, with a price tag of around $1.8 million price tag, saying its ability to help patients reach sustained transfusion independence justified its high price. Eli-cel was also previously approved in Europe as Skysona.

ISPE
DECEMBER 1, 2022
Winner of the ISPE Italy Affiliate’s Women in Pharma® Graziella Molinari Award in 2021, thanks to her project for the inclusion of #smart tags in a site production in order to improve not only efficiency, but also quality and compliance in a 4.0 trillion dollars in global turnover. Micaela Prati. perspective. .

pharmaphorum
APRIL 5, 2022
Immunocore now has approval on both side of the Atlantic for Kimmtrak – the first cancer therapeutic based on T cell receptor (TCR) technology – after getting a green light from the European Commission. The European population is meanwhile estimated at several thousand patients, across all stages, according to registry data.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?

Let's personalize your content