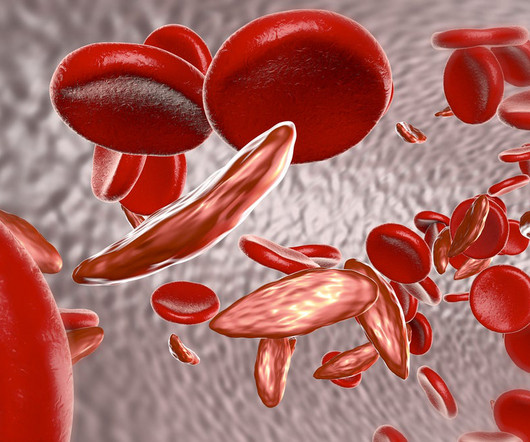
Upcoming sickle cell gene therapies cost effective at $2 million, says ICER
Pharmaceutical Technology
APRIL 13, 2023
Two gene therapies up for approval this year for sickle cell disease could be cost effective in some cases at a $2 million price point, based on a draft evidence report published by the Institute for Clinical and Economic Review (ICER). The standard of care for the condition includes blood transfusions, hydroxyurea and iron chelation.














Let's personalize your content