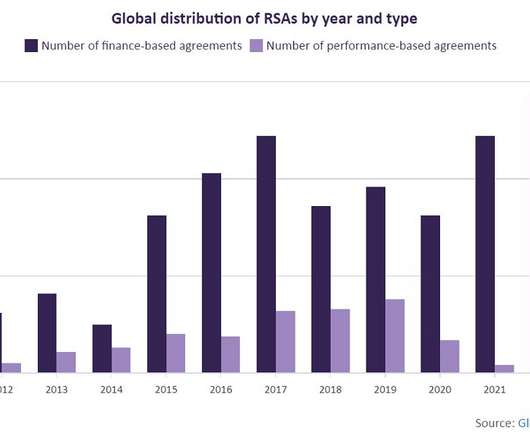
Risk-sharing agreements are growing at a rate of 24%
Pharmaceutical Technology
FEBRUARY 10, 2023
This is not the first treatment to come with a high price tag. On November 22, 2022, the FDA approved CSL Behring’s Hemgenix (etranacogene dezaparvovec), the first gene therapy treatment for hemophilia B, with a staggering manufacturer price of $3.5


















Let's personalize your content