Pfizer's Supreme Court challenge of US kickback law is 'far-fetched,' HHS says
Fierce Pharma
DECEMBER 15, 2022
Pfizer's Supreme Court challenge of US kickback law is 'far-fetched,' HHS says. Thu, 12/15/2022 - 14:50.

 law us-supreme-court
law us-supreme-court 
Fierce Pharma
DECEMBER 15, 2022
Pfizer's Supreme Court challenge of US kickback law is 'far-fetched,' HHS says. Thu, 12/15/2022 - 14:50.

Pharmaceutical Technology
FEBRUARY 22, 2023
Amgen could be the winner of a high-stakes patent spat with Sanofi as tensions run high weeks before the March 27 US Supreme Court hearing. While some courts were previously on Sanofi’s side, it is possible that Amgen’s case could win this time, some legal experts say. However, the case was brought back.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
MAY 19, 2023
The US Supreme Court has unanimously voted in favour of Sanofi and Regeneron in a years-long legal feud with Amgen over the potential patent infringement surrounding the companies’ anti-cholesterol drugs. After previously receiving the arguments from both companies on March 27, the Supreme Court delivered its verdict on May 18.

pharmaphorum
JANUARY 12, 2023
The Attorney General of Alabama has threatened to use an obscure law passed in 2006 to prosecute women who terminate a pregnancy using pharmacological means, shortly after the FDA made access to the drugs easier. ” The post Women in Alabama could face prosecution over use of abortion pills appeared first on.

pharmaphorum
JULY 6, 2022
A court in West Virginia has ruled that US drug distributors McKesson, AmerisourceBergen and Cardinal Health are not responsible for fuelling the opioid crisis that has claimed thousands of lives in the state. District court judge David Faber rejected a call by prosecutors to force the distributors to pay $2.5

The FDA Law Blog
AUGUST 21, 2023
Livornese — I saw the sign…and the answer is no—FDA-approved labeling apparently is not enough under state failure-to-warn laws, according to certain courts. A recent state law failure-to-warn case in the SDNY makes that very point.
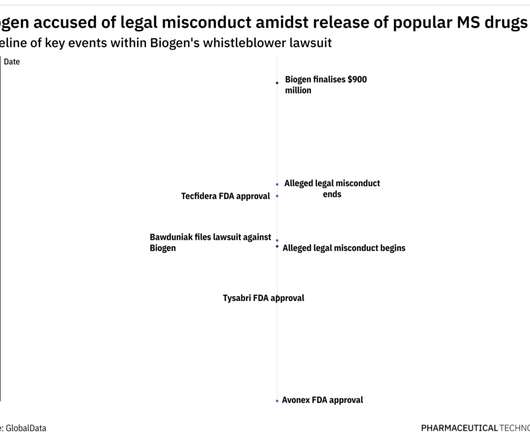
Pharmaceutical Technology
OCTOBER 5, 2022
As per the US Department of Justice, Biogen paid remuneration in the form of training and consulting fees and speaker honoraria, to induce physicians to prescribe the company’s drugs, in violation of the Anti-Kickback Statute. Under this law, relators such as Bawduniak can file lawsuits on behalf of the government.
Let's personalize your content