FDA approves Sanofi and Regeneron’s Dupixent for bullous pemphigoid
Pharmaceutical Technology
JUNE 25, 2025
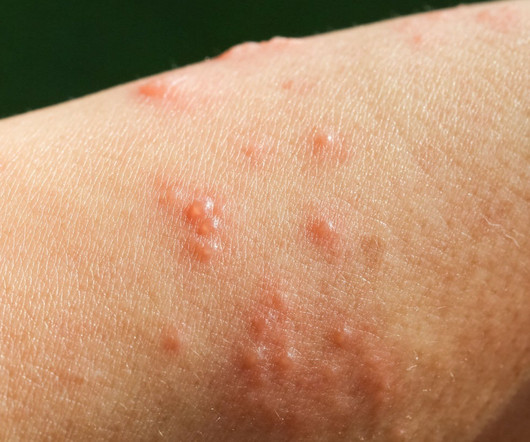
The US Food and Drug Administration (FDA) has approved Sanofi and Regeneron’s Dupixent (dupilumab) as a treatment option for adults with bullous pemphigoid (BP), a condition that predominantly affects the elderly. In February 2025, the FDA accepted Dupixent’s supplemental biologics licence application (sBLA) for priority review.





































Let's personalize your content