Rapid COVID immune status test launched in UK, Ireland
pharmaphorum
JULY 26, 2021
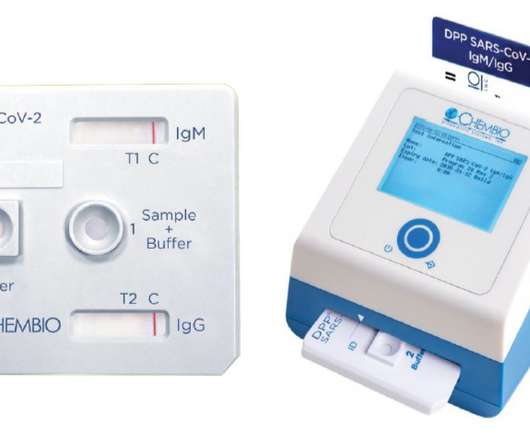
While there are plenty of tests already available that can give a yes/no reading of whether antibodies are present, the Chembio test measures the level of antibodies as well, so can tell whether a patient is likely to be protected against the coronavirus. The post Rapid COVID immune status test launched in UK, Ireland appeared first on.






























Let's personalize your content