Alkermes looks to fill cancer treatment gaps with new immune pathways
PharmaVoice
JULY 14, 2022
The company’s chief medical officer discusses its lead candidate and how it fits into wider oncology trends.

PharmaVoice
JULY 14, 2022
The company’s chief medical officer discusses its lead candidate and how it fits into wider oncology trends.
Pharmacy Times
JULY 14, 2022
The objective of this study is to evaluate the use of low-dose pioglitazone, without relying on high doses of insulin, by enhancing sensitivity as a suitable, cost-effective strategy compared to larger insulin doses in patients with limited access to care.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
JULY 15, 2022
Insulin prices made the headlines again as California governor Gavin Newsom announced plans on 7 July for the state to manufacture low-cost insulin. In a budget change proposed in February and confirmed in May , California’s Department of Health Care Access and Information (HCAI) requested a one-time investment of $100 million for Newsom’s CalRx Biosimilar Insulin initiative.

pharmaphorum
JULY 15, 2022
Akili looks like it could bring another digital therapeutic (DTx) to market after a clinical trial backed the efficacy of its AKL-T01 in patients with the autoimmune disorder systemic lupus erythematosus (SLE). The PureTech group company – which scored the first FDA and EMA approvals for a DTx for attention-deficit hyperactivity disorder (ADHD) – is developing AKL-T01 to tackle cognitive impairments that can affect people with SLE.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

PharmaVoice
JULY 13, 2022
Elizabeth Izard Apelles is finding innovative ways to champion rare disease patients through an emerging platform from Honeycomb Health.
Pharmacy Times
JULY 11, 2022
The non-estrogen pill was first approved by the FDA in 1973.
Pharmacy Technician Pulse brings together the best content for pharmacy technicians from the widest variety of industry thought leaders.

Find Your Script
JULY 9, 2022
Watch & Subscribe on our YouTube Channel ! Today we are talking about why I chose pharmacy over medicine. Here the breakdown, the questions to consider and how to make an informed decision that aligns with your life. Stay until the end where I share the best step to take. . Hi, I’m Dr. Jessica Louie and I’m an Associate Professor, Board-Certified Critical Care Pharmacist and entrepreneur.

PharmaVoice
JULY 11, 2022
Deloitte's senior manager of R&D and regulatory practices explains how technology and strategy must converge to smooth out the bulky drug approval process.
Pharmacy Times
JULY 11, 2022
Christopher Fine, MD, FACC, a cardiologist at National Jewish Health, discusses how pharmacists can support patients with cancer who have a much higher risk of heart disease.

pharmaphorum
JULY 15, 2022
Pfizer’s tyrosine kinase inhibitor Xalkori has picked up a fourth approval from the FDA, adding a new use in the treatment of a rare form of benign tumour that typically affects children and young adults. Xalkori (crizotinib) has been cleared for inflammatory myofibroblastic tumours (IMT), which appear in organs like the lung, stomach, bladder or liver.

Speaker: Chris Antypas and Josh Halladay
Access to limited distribution drugs and payer contracts are key to pharmacy expansion. But how do you prepare your operations to take the next step? Meaningful data: Collect and share clinical data regarding outcomes, utilization, and more Reporting: Limited distribution models require efficient tracking and reporting systems Workflows: Align workflows with specific pharma and payer contractual requirements For in-depth, expert insights on pharmacy expansion, watch this webinar from Inovalon.

European Pharmaceutical Review
JULY 12, 2022
According to a recent survey of patient groups, Roche, Pfizer, ViiV Healthcare and Gilead Sciences are among the top companies for implementing and reporting progress on environmental, social, governance (ESG) activities. However the survey of 1,500 patient groups also found that 55 percent were not aware of pharma’s ESG programmes, suggesting pharma needs to better include and consider the patient perspective in establishing their ESG goals.

PharmaVoice
JULY 14, 2022
The pharma giant’s ‘Depression looks like me’ campaign assembles a ‘one stop shop’ of resources to help combat high rates of severe depression in the LGBTQ community.

Pharmacy Times
JULY 10, 2022
These clear-cut guidelines help pharmacists making sure the patients are getting the right drug to set them up for success and prevent problems down the road.

Pharmaceutical Technology
JULY 12, 2022
Vertex Pharmaceuticals has signed a definitive agreement for the acquisition of biotechnology company ViaCyte in a deal totalling $320m in cash. ViaCyte focuses on offering new stem cell-derived cell replacement therapies as a functional cure for type 1 diabetes (T1D). Through the acquisition, Vertex plans to advance its potentially curative VX-880 programmes in T1D.

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.

European Pharmaceutical Review
JULY 12, 2022
Microbes developing resistance to disinfectants is a major emerging problem in pharmaceutical manufacturing cleanrooms and, if left unchecked, could present a threat to drug quality and, therefore, human health. More than 480 warning letters solely related to the failure to produce aseptic conditions because of deficient cleaning and disinfection systems for cleanrooms and their equipment, were issued by regulators between 2013 to 2018.

PharmaVoice
JULY 13, 2022
How the chief commercial and corporate strategy officer is embracing a ‘full-cup’ philosophy to build a better leadership toolbox.
Pharmacy Times
JULY 14, 2022
Patient education, naloxone training, and monitoring programs make a difference.

Pharmaceutical Technology
JULY 15, 2022
Despite entering its fourth decade of availability, in vitro fertilization (IVF), a medical technique used to facilitate the conception of a baby for those facing fertility problems, remains an elusive dream for many. Artificial intelligence (AI) could change this, with many researchers looking to harness the technology to increase IVF’s reliability, affordability, and availability.

Advertisement
Are you still using workarounds to manage your daily operations? To achieve peak performance, it's time to explore other options for specialty and infusion pharmacy software. Streamline pharmacy operations and improve clinical performance with automated processing, real-time data exchange, and electronic decision support. Download this helpful infographic to: Drive efficiency and patient adherence from referral receipt to delivery and ongoing care – all with our Pharmacy Cloud.
European Pharmaceutical Review
JULY 14, 2022
A new pilot project by the European Medicines Agency (EMA) will assess whether the analysis of ‘raw data’ from clinical trials improves the evaluation of marketing authorisation applications (MAAs) for new medicines as well as post-authorisation applications. The project will also evaluate the practicality of such submissions and analyses. Raw data constitutes individual patient data from clinical studies in electronic structured format that is directly accessible for analysis and visualisation.

PharmaVoice
JULY 15, 2022
The incoming CEO aims to build on the buzz around the company’s leading-edge gene therapy tech.

Pharmacy Times
JULY 12, 2022
Study results show that there is a link between leaving the television on, night lights, and smart phones and higher disease rates.
Pharmaceutical Technology
JULY 15, 2022
Health Canada has granted approval for the usage of Moderna’s messenger RNA (mRNA) Covid-19 vaccine, Spikevax, in a 25µg two-dose regimen for active immunisation to prevent Covid-19 in children aged six months to five years. So far, children aged below five years were not eligible to receive the Covid-19 vaccine in Canada. The two-dose initial vaccine regimen in children of this age group is completed in one month, which is the same dosing schedule in adults, adolescents and children aged above

European Pharmaceutical Review
JULY 15, 2022
The pharmaceutical sector is not typically seen as a highly polluting, ‘heavy industry’ but it is far from green. In its 2021 report Delivering a ‘Net Zero’ National Health Service , the UK’s NHS attributes as much as a quarter of its carbon footprint to medicines. A deep carbon footprint is a common hallmark of energy intensive manufacturing processes – and the manufacture of pharmaceuticals is no exception.

PharmaVoice
JULY 11, 2022
Phil Brown, Dermavant’s chief medical officer, discusses VTAMA — the first topical cream approved for psoriasis in 25 years.

Pharmacy Times
JULY 13, 2022
The FDA's current way of inspecting facilities for quality control is badly outdated and is politically unworkable, unreliable, and unsafe.

pharmaphorum
JULY 13, 2022
New York’s attorney general has accused Teva of lying to evade responsibility for its alleged role in fuelling the opioid epidemic in the state and is asking for a case against the company to be reopened. AG Letitia James says the Israeli drugmaker “made significant and intentional misrepresentations” to her office and the court about its involvement with its US subsidiary in order to evade legal action and accountability.
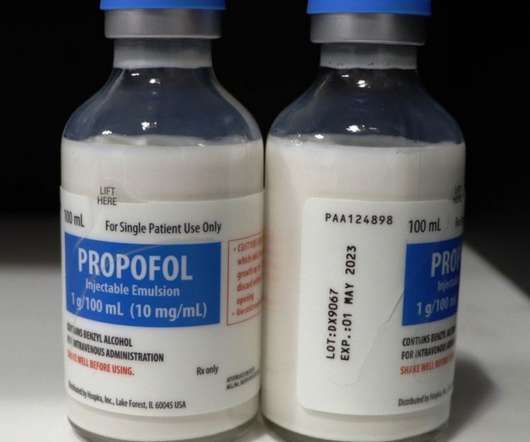
European Pharmaceutical Review
JULY 14, 2022
Pfizer’s Hospira is voluntarily recalling one lot of Propofol Injectable Emulsion, USP (containing benzyl alcohol), 100ml Single Patient Use Glass Fliptop Vial; lot DX9067, to the user level due to a visible particulate observed in a single vial during annual examination of retain samples. According to a statement released by Pfizer, patients receiving the impacted product are at risk of experiencing life-threatening adverse clinical effects, including blockage of blood vessels (with potential t

Pharma Tutor
JULY 12, 2022
FACTOR AFFECTING ON THE DANDRUFF GROWTH AND THE IMPACT OF NATURAL HERBS AS AN ANTI-DANDRUFF AGENT. About Authors. Avdesh Thassu. MPharm.(Nat. Chem.); MBA (Mkt.). Associate Vice President. Global Regulatory Affairs, Emami Limited, 7044455199. athassu@hotmail.com. Chandra Mohan Nandi. MSc.(Med. Chem.). Deputy Manager, Global Regulatory Affairs, Emami Limited, 9051706947. chandramohannandi@yahoo.in. admin.

Pharmacy Times
JULY 12, 2022
The American Diabetes Association guidelines have self-care and lifestyle recommendations for patients and health care professionals.

pharmaphorum
JULY 13, 2022
Roche’s Genentech unit has gone all in on its collaboration with UK biotech Bicycle Therapeutics, taking up an option on a second additional target in the area of cancer immunotherapy. Bicycle’s bicyclic peptide drug discovery platform is designed to produce drug candidates that have targeting properties like antibodies but are much smaller molecules, making manufacturing and dosing easier.

PharmExec
JULY 15, 2022
Podcast recognized for its excellence in apps and podcasts category.

European Pharmaceutical Review
JULY 15, 2022
A report assessing the current climate for antimicrobial innovation in Brazil, India, and South Africa, reveals that removing regulatory hurdles to accelerate the approval of new antibiotics will help to improve their development and access. Antimicrobial resistance (AMR) is a growing challenge to the healthcare sector, as antibiotics are a linchpin of modern medicine.

Pharmacy Times
JULY 12, 2022
Vibhuti Arya Amirfar, PharmD, MPH, discusses building individual and systems resilience to dismantle structural racism in the pharmacy field.
Let's personalize your content