Poll: Majority of U.S. voters approve of giving Medicare drug price negotiation authority
Fierce Healthcare
SEPTEMBER 29, 2021
Poll: Majority of U.S. voters approve of giving Medicare drug price negotiation authority. rking. Wed, 09/29/2021 - 15:53.

Fierce Healthcare
SEPTEMBER 29, 2021
Poll: Majority of U.S. voters approve of giving Medicare drug price negotiation authority. rking. Wed, 09/29/2021 - 15:53.

pharmaphorum
SEPTEMBER 29, 2021
Amazon’s push into healthcare takes many forms, and one is decidedly biotech-like – running an accelerator to identify and support startups with promising technology platforms. The online commerce giant’s first accelerator kicked off in June, focusing on digital health companies, and 427 applications from 31 countries around the world have now been winnowed down to 10 finalists.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Outsourcing Pharma
SEPTEMBER 30, 2021
A study by Charles River finds patients believe the overall quality of healthcare would increase if stakeholders across the life sciences collaborated more.

Pharma Times
SEPTEMBER 28, 2021
Informa Pharma Intelligence (IPI) has found that some drug firms may withdraw up to 90% of their products from Northern Ireland, once the Brexit grace period has passed.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Fierce Healthcare
SEPTEMBER 28, 2021
Amazon taps VR, voice assistant startups and 8 others for inaugural digital health accelerator. hlandi. Tue, 09/28/2021 - 14:54.

pharmaphorum
SEPTEMBER 29, 2021
A research project between Bayer and digital health company Huma will use artificial intelligence to detect lung cancer in CT scans – and determine which type a patient has, in order to direct treatment. AI is already being applied by a number of groups, with some studies indicating it can even be more effective than trained radiologists in detecting subtle patterns that indicate the presence of tumours.
Pharmacy Technician Pulse brings together the best content for pharmacy technicians from the widest variety of industry thought leaders.

Pharma Mirror
SEPTEMBER 30, 2021
As the longest established tablet compression tooling manufacturer in the world, I Holland is celebrating its 75th anniversary. The company has reached this remarkable milestone due to their commitment to research, development and investment which has established the company as the pre-eminent supplier of punches and dies to producers of tablets across the globe.

Fierce Healthcare
SEPTEMBER 30, 2021
Right-wing physicians profit off of fake COVID-19 treatments, report finds. agliadkovskaya. Thu, 09/30/2021 - 16:03.
pharmaphorum
SEPTEMBER 28, 2021
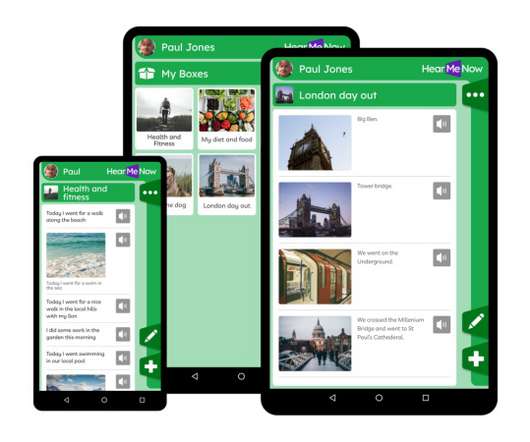
An app to support people with cognitive disabilities, developed with clinicians at Barts Health NHS Trust, has started to be made available more widely to patients. The Hear Me Now app developed with UCLPartners and software company Maldaba is designed to improve quality of care for people with cognitive impairment, specifically adults with a learning disability.
Outsourcing Pharma
SEPTEMBER 28, 2021
Q BioMed reports it expects candidate MAN-19, intended to treat COVID-19-related Acute Respiratory Distress Syndrome, to enter trials early next year.

Speaker: Chris Antypas and Josh Halladay
Access to limited distribution drugs and payer contracts are key to pharmacy expansion. But how do you prepare your operations to take the next step? Meaningful data: Collect and share clinical data regarding outcomes, utilization, and more Reporting: Limited distribution models require efficient tracking and reporting systems Workflows: Align workflows with specific pharma and payer contractual requirements For in-depth, expert insights on pharmacy expansion, watch this webinar from Inovalon.

Pharma Mirror
SEPTEMBER 30, 2021
SINGAPORE; The Agency for Science, Technology and Research (A*STAR) has developed an Automated Vaccine Inoculation Dispenser (AVID) system, which replaces the manual step of filling injection syringes with vaccine liquid. AVID is customised for the vaccination centres which are set up by the Ministry of Health and operated by private healthcare providers AVID was designed to address the labour-intensive steps of the vaccination process to reduce the workload of healthcare providers, increase acc

Fierce Healthcare
SEPTEMBER 30, 2021
VR can help underserved patients, but reimbursement challenges stymie broader adoption, study finds. hlandi. Thu, 09/30/2021 - 11:14.

pharmaphorum
SEPTEMBER 27, 2021
Takeda has chalked up another milestone for its Alofisel cell therapy for a complication of Crohn’s disease, becoming the first allogeneic stem cell therapy to be approved in Japan. Alofisel (darvadstrocel) has been cleared by the Ministry of Health, Labour and Welfare (MHLW) for the treatment of complex perianal fistulas in patients with non-active or mildly active luminal Crohn’s disease who have not responded to conventional therapies.
Outsourcing Pharma
SEPTEMBER 27, 2021
The protease inhibitor PF-07321332 (co-administered with ritonavir) is designed for patients exposed to (or showing the first signs of) the COVID-19 virus.

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.

Pharma Marketing Network
SEPTEMBER 29, 2021
John Lineen is a senior executive with strong marketing, digital, analytics, and CX experience in the pharmaceutical industry. John’s specialty is managing, launching, measuring, and optimizing large-scale digital and omnichannel programs. He uses his global corporate (9+ years at Bristol Myers Squibb, GlaxoSmithKline), digital agency (12+ years @ Digitas Health), consulting (McKinsey & Company), start-up, and engineering background to deliver engaging content and services that help HCPs and

Fierce Healthcare
SEPTEMBER 29, 2021
Medicare Advantage premiums to decline slightly in 2022, Part D to rise by nearly 5%. rking. Wed, 09/29/2021 - 14:49.

pharmaphorum
SEPTEMBER 29, 2021
Oliver Stohlmann’s Corporate Survival Hacks series draws on his experiences of working in local, regional and global life science communications to offer some little tips for enjoying a big business career. In this column he addresses the appropriate use of email and how to avoid nightmares in electronic communication. “My fiancée had bought me a string tanga birthday present.

Outsourcing Pharma
SEPTEMBER 28, 2021
Conducted by patient services specialist Greenphire, the survey checks in with contract research organizations and shows how they are approaching obstacles.

Advertisement
Are you still using workarounds to manage your daily operations? To achieve peak performance, it's time to explore other options for specialty and infusion pharmacy software. Streamline pharmacy operations and improve clinical performance with automated processing, real-time data exchange, and electronic decision support. Download this helpful infographic to: Drive efficiency and patient adherence from referral receipt to delivery and ongoing care – all with our Pharmacy Cloud.

Pharma Marketing Network
SEPTEMBER 29, 2021
Timothy Glennon . The post Timothy Glennon appeared first on Pharma Marketing Network.

Fierce Healthcare
SEPTEMBER 27, 2021
CVS Caremark takes aim at health inequities, starting with sickle cell, HIV. pminemyer. Mon, 09/27/2021 - 15:55.

pharmaphorum
SEPTEMBER 28, 2021
Prospects for Valneva’s COVID-19 vaccine are up in the air, but another candidate partnered with Pfizer looks increasingly like it could fill the void of an effective shot for Lyme disease. New phase 2 data with their VLA15 vaccine shows that antibody responses from an initial three-dose regimen are initially high, although they start to decline after 18 months in adult subjects.
Outsourcing Pharma
OCTOBER 1, 2021
The pharma firm reports molnupiravir was shown to cut risk of hospitalization or death by 50% compared to placebos in a positive interim Phase III analysis.

Pharma Mirror
SEPTEMBER 28, 2021
Unfair competition arising in the pharmaceutical markets and the presence of a shadow sector require the protection of the brand’s interests and confirmation of quality for the end customer. In recent years, the reaction to this has been the emergence of intercorporate methods of protecting goods from counterfeiting. The regulators of specific countries of national projects have started to control counterfeit and falsification by creating complex systems that verify each unit of goods.

Fierce Healthcare
SEPTEMBER 29, 2021
Labor shortages in healthcare expected to rise as demand grows, report finds. agliadkovskaya. Wed, 09/29/2021 - 16:45.

pharmaphorum
OCTOBER 1, 2021
Already a major player in atopic dermatitis with Dupixent, Sanofi looked to expand its position in the category earlier this year when it bought Kymab and its lead drug KY1005 for the skin disorder for $1.1 billion upfront. Now, it says the drug has delivered “exciting” results in a proof-of-concept trial. KY1005 – now renamed amlitelimab – is one of a handful of new drugs targeting OX40 ligand, an immune system regulator delivered by intravenous infusion once every 28 days.

Outsourcing Pharma
SEPTEMBER 30, 2021
A leader from Swift Medical describes the companyâs skin and wound management technology, and how their tech empowers patient capture of vital information.

Pharma Marketing Network
SEPTEMBER 28, 2021
Name *. First. Last. Title * Company * Email *. Name This field is for validation purposes and should be left unchanged. The post PMN Innovation Summit 2021 appeared first on Pharma Marketing Network.

Fierce Healthcare
OCTOBER 1, 2021
Humana launching 72 new Medicare plans for 2022. pminemyer. Fri, 10/01/2021 - 11:09.

pharmaphorum
SEPTEMBER 30, 2021
In this instalment of EPG Health’s HCP engagement series, Georga Cottle discusses how case-based learning connects medical theory to medical practice. Medical education doesn’t stand still. The acute demands on today’s healthcare systems require ever-more effective learning opportunities for healthcare professionals (HCPs) and offer pharma companies valuable chances to support evidence-based clinical practice.

Outsourcing Pharma
SEPTEMBER 27, 2021
A leader from Firma Clinical Research advises that while using RWD can be daunting, putting the data to use can lead to a number of notable advantages.

Fuld
SEPTEMBER 28, 2021
Background. Within the retail sector “hero items” or “destination items” are the standout products that attract customers to specific stores and drive additional sales, whether those are discretionary purchases or the weekly supermarket shop. The challenge for retailers is how to effectively measure performance to successfully identify these items, and what marketing strategy and tactics they should then apply to attract more customers and drive sales.

Fierce Healthcare
OCTOBER 1, 2021
Providers cry foul over new surprise billing rule's arbitration process. rking. Fri, 10/01/2021 - 16:39.

pharmaphorum
SEPTEMBER 29, 2021
AstraZeneca’s newly acquired Alexion will buy the remaining equity in rare disease specialist Caelum Biosciences in a deal that could be worth up to $500 million. The deal gives the Anglo-Swedish drugmaker complete control of Caelum’s late-stage medicine for light chain (AL) amyloidosis, a potentially fatal disease that causes the rogue protein amyloid to build up in organs like the heart and kidneys.
Let's personalize your content