Interactive Case Studies: May 2023
Pharmacy Times
MAY 29, 2023
Cases address gastrointestinal issues and alcohol abuse.
Pharmacy Times
MAY 29, 2023
Cases address gastrointestinal issues and alcohol abuse.
STAT
MAY 29, 2023
For busy primary care physicians like me, an annual physical with a 27-year-old male is a blessing. Since we’re always running late, a quick visit with a young healthy adult offers the rare chance to get back on schedule. But I didn’t give my own doctor that chance to get back on schedule when I went to see him for my physical a few months ago.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

European Pharmaceutical Review
MAY 29, 2023
A neurosteroid drug was one of two medicines recommended for approval at the Committee for Medicinal Products for Human Use (CHMP) ’s May 2023 meeting. Ztalmy (ganaxolone) received a positive opinion for epileptic seizures associated with cyclin-dependent kinase-like 5 deficiency disorder. This genetic condition is defined by seizures starting during infancy.
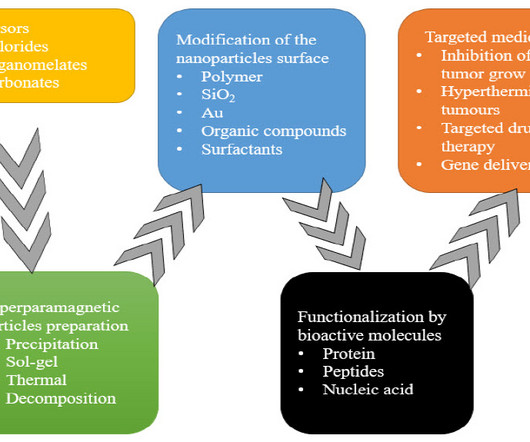
Pharma Tutor
MAY 29, 2023
A REVIEW OF MAGNETIC NANOPARTICLES IN TARGETED DRUG DELIVERY admin Mon, 05/29/2023 - 15:04 About Authors CHETNA MODI 1 *, VINIT MODI 2 , SHWETA RAY 2 , PIYUSH NARIYA 2 , VARSHA GADHVI 2 1 Professor, Department of Pharmaceutics, Anand Pharmacy College, Anand, Gujarat, India. 2 Research Scholar, Department of Pharmaceutics, Anand Pharmacy College, Anand, Gujarat, India. * chetnamodi306@gmail.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven
pharmaphorum
MAY 29, 2023
EU approval for Novartis’ sickle cell drug set to be pulled Phil.

The People's Pharmacy
MAY 29, 2023
If you are out in the woods or fields this summer, you’ll want to be wary of a noxious weed that can cause a terrible itchy rash. We are referring, of course, to poison ivy. Poison oak and poison sumac also produce the same irritating resin, urushiol. Prevention is the best medicine. Don’t touch poison ivy or poison oak if you can possibly avoid it.
Pharmacy Technician Pulse brings together the best content for pharmacy technicians from the widest variety of industry thought leaders.

The People's Pharmacy
MAY 29, 2023
Post-operative nausea can be very troubling. People who have had any type of abdominal surgery definitely want to avoid throwing up. In addition to the usual unpleasantness, vomiting after surgery could be painful or even threaten the stitches.Even those who have had other types of surgery do not welcome nausea following surgery. One reader found that ginger made was extremely helpful in this situation.

Pharmacy Times
MAY 29, 2023
Cases discuss triglyceride levels, supplement use, and more.

pharmaphorum
MAY 29, 2023
SGLT latecomer Lexicon gets heart failure okay Phil.
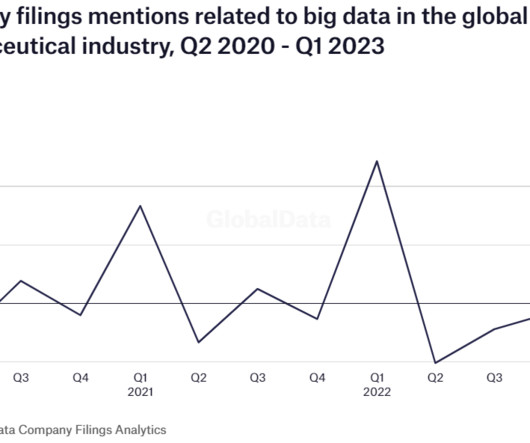
Pharmaceutical Technology
MAY 29, 2023
The global pharmaceutical industry experienced a 13% drop in company filings mentions of big data in Q1 2023 compared with the previous quarter, with the highest share accounted for by Novartis with 185% year-on-year increase, according to GlobalData’s analysis of over 603 pharmaceutical company filings. The big data landscape is rapidly evolving and pharma companies are increasingly integrating big data analytics into their value chains.

Speaker: Chris Antypas and Josh Halladay
Access to limited distribution drugs and payer contracts are key to pharmacy expansion. But how do you prepare your operations to take the next step? Meaningful data: Collect and share clinical data regarding outcomes, utilization, and more Reporting: Limited distribution models require efficient tracking and reporting systems Workflows: Align workflows with specific pharma and payer contractual requirements For in-depth, expert insights on pharmacy expansion, watch this webinar from Inovalon.

The People's Pharmacy
MAY 29, 2023
June is Brain Health Month, which is great timing because CocoaVia TM is also celebrating 1 year since the launch of Memory & Focus – its first and only formula with a unique blend of ingredients for Brain Health. Memory & Focus supports 5 areas of brain performance including complex attention, visual memory, executive cognitive function, word recall and long-term memory.

Pharmaceutical Technology
MAY 29, 2023
The global pharmaceutical industry experienced a 17% rise in company filings mentions of blockchain in Q1 2023 compared with the previous quarter, with the highest share accounted for by Bayer, according to GlobalData’s analysis of over 60 pharmaceutical company filings. GlobalData’s Blockchain Market Size, Share, Trends, and Segment Forecast to 2030 report offers insights on application of blockchain in healthcare and other key sectors.

PharmaShots
MAY 29, 2023
Shots: The MHLW has approved Ultomiris (C5 complement inhibitor) for the prevention of relapses in patients with AQP4 Ab+ neuromyelitis optica spectrum disorder (NMOSD) incl. neuromyelitis optica The approval was based on the P-III trial (CHAMPION-NMOSD) evaluating Ultomiris in 58 patients across North America, the EU, Asia-Pacific & Japan. The trial met its 1EPs of time to first on-trial relapse as confirmed by an independent adjudication committee, zero relapses were seen with a median tre
Pharmaceutical Technology
MAY 29, 2023
The US Food and Drug Administration (FDA) has accepted Iovance Biotherapeutics’ biologics license application (BLA) for lifileucel to treat advanced melanoma. Lifileucel is a tumour infiltrating lymphocyte (TIL) therapy developed to treat advanced melanoma patients who advanced on or after prior anti-PD-1/L1 therapy and targeted therapy. It has been granted priority review by the FDA, with a target action date of 25 November this year, under the Prescription Drug User Fee Act (PDUFA).

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.

PharmaShots
MAY 29, 2023
Shots: The EMA’s CHMP adopted the positive opinion recommending the approval of Opdivo + Pt-based CT for resectable NSCLC who are at a high risk of recurrence in adult patients with tumor cell PD-L1 expression ≥1% The opinion was based on the P-III trial (CheckMate -816) evaluating Opdivo (360mg, q3w) + CT vs CT alone which showed an improvement in EFS & pCR with 3 cycles of Opdivo + CT vs CT alone The safety profile was consistent with prior reported studies while the 3yr. data sho
Pharmaceutical Technology
MAY 29, 2023
Novavax has received positive opinion from the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) for full marketing authorisation (MA) of its Covid vaccine, Nuvaxovid (NVX-CoV2373) , in the European Union (EU). A protein-based vaccine, Nuvaxovid has been recommended for MA for use as a primary series in individuals of the age 12 years and above and as a booster in those aged 18 years and older to prevent Covid-19.

PharmaShots
MAY 29, 2023
Shots: The companies collaborated to discover & develop novel therapies for patients with neurodegenerative diseases incl. Parkinson's disease The collaboration will combine companies’ expertise and resources in developing NurrOn's ATH-399A & other compounds targeting Nurr1 for neurodegenerative disorders. The P-I trial of ATH-399A is expected to be initiated in 2023 The companies also continue to advance the therapy in other neurodegenerative diseases.

Pharmaceutical Technology
MAY 29, 2023
The global pharmaceutical industry experienced a 38% drop in company filings mentions of virtual care in Q1 2023 compared with the previous quarter, with the highest share accounted for by ICON with 25% year-on-year decrease, according to GlobalData’s analysis of over 88 pharmaceutical company filings. GlobalData’s Telehealth Market Size by Segments, Share, Trends, and Forecast, 2022-2030 report provides key insights into business strategies, trends driving the telehealth market and granul

Advertisement
Are you still using workarounds to manage your daily operations? To achieve peak performance, it's time to explore other options for specialty and infusion pharmacy software. Streamline pharmacy operations and improve clinical performance with automated processing, real-time data exchange, and electronic decision support. Download this helpful infographic to: Drive efficiency and patient adherence from referral receipt to delivery and ongoing care – all with our Pharmacy Cloud.

PharmaShots
MAY 29, 2023
Shots: This is a P-Ib dose-expansion study, evaluating the safety, tolerability, PK/PD, and preliminary efficacy of porustobart/HBM4003 (0.45 mg/kg) + toripalimab (240 mg Q3W) in patients (n=28) with advanced HCC and other solid tumors Patients (n=16) who failed previous anti-VEGFR treatment while have not received anti-PDL1 treatment (Cohort 1) and patients (n=12) who failed previous anti-PD-L1 and anti-VEGFR treatments (Cohort 2) As of 9 Dec 2022, the median follow-up time was 3.6mos. while th

Pharmaceutical Technology
MAY 29, 2023
The global pharmaceutical industry experienced a 24% drop in company filings mentions of regenerative medicine in Q1 2023 compared with the previous quarter, with the highest share accounted for by Sarepta Therapeutics with 20% year-on-year decrease, according to GlobalData’s analysis of over 578 pharmaceutical company filings. GlobalData’s Regenerative Medicine in Medical thematic intelligence report provides an overview of the current landscape, including healthcare, technology, regulato

PharmaShots
MAY 29, 2023
Shots: David first talked about Clarivate’s Highly Cited Researchers 2022 list. He said that the list picks out 6,938 individuals at universities, research institutes, and commercial organizations across 69 countries/regions He also elucidated the methodology used by Clarivate to create the list and how the pharma companies can benefit from this list to strengthen their strategies The interview showcases how Clarivate is working to provide actionable information and insights that bring new

Pharmaceutical Technology
MAY 29, 2023
The global pharmaceutical industry experienced a 30% drop in company filings mentions of microbiome in Q1 2023 compared with the previous quarter, with the highest share accounted for by Novozymes with 9% year-on-year decrease, according to GlobalData’s analysis of over 50 pharmaceutical company filings. GlobalData’s Microbiome-Targeting Therapeutics in Infectious Diseases – Thematic Research report provides information on market classification by therapy and technologies, regulatory and m

PharmaShots
MAY 29, 2023
Shots: The P-III trial evaluating Imfinzi + Pt-based CT, followed by Imfinzi with Lynparza or Imfinzi alone as maintenance therapy vs Pt-based CT alone in 699 patients at 253 study locations across 22 countries incl. the US, EU, South America & Asia The results showed a significant & clinical improvement in PFS, greater benefits were seen with the combination of Lynparza + Imfinzi as maintenance treatment while OS data were immature & favorable trends were reported for both a treatme

Pharmaceutical Technology
MAY 29, 2023
The global pharmaceutical industry experienced a 27% drop in company filings mentions of future of work in Q1 2023 compared with the previous quarter, with the highest share accounted for by Moderna with 13% year-on-year decrease, according to GlobalData’s analysis of over 419 pharmaceutical company filings. GlobalData’s Future of Work – Thematic Research report explains how the theme future of work is impacting the workforce across different sectors, it presents key technology, macroecono

PharmaShots
MAY 29, 2023
Shots: The US FDA has accepted the BLA for lifileucel to treat patients with advanced melanoma & granted Priority Review with an expected PDUFA on Nov 2023. The submission was based on the (C-144-01) trial who progressed on or after prior anti-PD-1/L1 therapy and targeted therapy The results showed ORR (31.4%) with 9 CRs and 39 PRs, the median time to best response was 1.5mos. and responses deepened over time, m-DOR (not reached) at 36.5mos. median study follow-up, 41.7% of responses lasted

The People's Pharmacy
MAY 29, 2023
Cimetidine ( Tagamet ) was the first prescription H2 antagonist to ease symptoms of heartburn and heal ulcers. It was introduced in the U.S. in 1977 as a prescription medication. This drug became a giant success until ranitidine ( Zantac ) came along and captured the market. Now, ranitidine is gone because of concerns about nitrosamine contamination.

European Pharmaceutical Review
MAY 29, 2023
Recently, the European Medicines Agency (EMA) took the lead in pushing for process improvements using technologies already established in other manufacturing sectors. One of these key technologies is automation, a recent paper explained. The authors offered clarification on the regulation and technologies to enable the cultural shift in the pharmaceutical environment, particularly within aseptic manufacturing and the use of robotics to fulfil good manufacturing practice (GMP).
Pharmaceutical Technology
MAY 29, 2023
Lexicon Pharmaceuticals (Lexicon) has received approval from the US Food and Drug Administration (FDA) for its Inpefa drug to treat heart failure. Inpefa is a once-daily oral tablet indicated as an inhibitor of sodium-glucose co-transporter type 2 (SGLT2) and type 1 (SGLT1). It is intended to lower the risk of cardiovascular death, urgent heart failure visit, and hospitalisation for heart failure.

The People's Pharmacy
MAY 29, 2023
Clonidine is an old drug by any standard. It was developed by Boehringer Ingelheim in the early 1960s to treat nasal congestion. The idea was to administer clonidine as nose drops. But the medication also lowered blood pressure and was introduced for the oral treatment of hypertension in 1966 ( Best Practice & Research Clinical Anaesthesiology , June, 2000 ).
Pharmaceutical Technology
MAY 29, 2023
FivepHusion has collaborated with Treehill Partners and Syneos Health to strategically progress development and commercialisation of its chemotherapeutic formulation Deflexifol in markets across the globe. Under the partnership, Treehill will support FivepHusion with operational, strategic and transactional expertise along with integrated business development.

Pharmaceutical Technology
MAY 29, 2023
The global pharmaceutical industry experienced an 18% drop in company filings mentions of orphan designated drugs in Q1 2023 compared with the previous quarter, with the highest share accounted for by Horizon Therapeutics with 50% year-on-year increase, according to GlobalData’s analysis of over 182 pharmaceutical company filings. GlobalData’s New Drug Approvals and Their Contract Manufacture report provides critical insight into the contract manufacturing organization (CMO) industry by an
Let's personalize your content