Biotech R&D spending has skyrocketed, and so have opportunities for savings
PharmaVoice
JULY 19, 2022
With R&D costs on the rise, biotechs are embracing new strategies and technologies to make spending more efficient.

PharmaVoice
JULY 19, 2022
With R&D costs on the rise, biotechs are embracing new strategies and technologies to make spending more efficient.

Pharmacy Times
JULY 19, 2022
Mark these products for mandatory patient education on the cartons and in the pharmacy computer system.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

PharmaVoice
JULY 19, 2022
John LaMattina has something to say about the industry — and he hopes patients are listening.

Pharmacy Times
JULY 19, 2022
Zonisade is the first and only FDA-approved oral liquid formulation of zonisamide, which helps patients with epilepsy who have difficulty swallowing tablets.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Pharmaceutical Technology
JULY 19, 2022
The US Food and Drug Administration (FDA) has granted approval for Incyte’s Opzelura (ruxolitinib) cream 1.5% as a topical treatment of nonsegmental vitiligo in adults and paediatric patients aged 12 years and above. Opzelura is a topical formulation of a Janus kinase (JAK) inhibitor. With the latest development, Opzelura has became the first treatment for repigmentation in patients with vitiligo to receive FDA approval.
Pharmacy Times
JULY 19, 2022
Treatment's approval marks exciting new development for patients with ES-SCLC to prevent CIM.
Pharmacy Technician Pulse brings together the best content for pharmacy technicians from the widest variety of industry thought leaders.
Pharmacy Times
JULY 19, 2022
Organizations such as the Appalachian Community Cancer Alliance are working to leverage technology and improve barriers to care.

pharmaphorum
JULY 19, 2022
Incyte’s Opzelura cream has become the first medical treatment approved to re-pigment the skin of people with vitiligo in the US, adding to its current use in atopic dermatitis. The FDA’s decision to clear the new indication for the topical JAK1/JAK2 inhibitor came after a priority review that was delayed by a request for additional data by the regulator, holding back a decision by three months.

Pharmacy Times
JULY 19, 2022
Pharmacies are so much more attuned to being able to use that technician to the top of his or her license and credential.
Pharmaceutical Technology
JULY 19, 2022
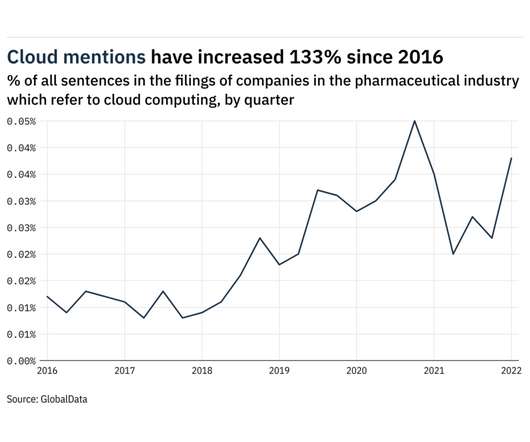
Mentions of cloud computing within the filings of companies in the pharmaceutical industry rose 65% between the final quarter of 2021 and the first quarter of 2022. In total, the frequency of sentences related to cloud computing between April 2021 and March 2022 was 133% higher than in 2016 when GlobalData, from whom our data for this article is taken, first began to track the key issues referred to in company filings.

Speaker: Chris Antypas and Josh Halladay
Access to limited distribution drugs and payer contracts are key to pharmacy expansion. But how do you prepare your operations to take the next step? Meaningful data: Collect and share clinical data regarding outcomes, utilization, and more Reporting: Limited distribution models require efficient tracking and reporting systems Workflows: Align workflows with specific pharma and payer contractual requirements For in-depth, expert insights on pharmacy expansion, watch this webinar from Inovalon.

Pharmacy Times
JULY 19, 2022
Indications for adalimumab include ankylosing spondylitis, Crohn disease, chronic plaque psoriasis, juvenile idiopathic arthritis, moderate to severe rheumatoid arthritis, psoriatic arthritis, and ulcerative colitis.

pharmaphorum
JULY 19, 2022
China’s Simcere Pharmaceutical has been granted approval in its home market for Cosela, a drug designed to limit the side effects of cancer chemotherapy, partnered with US biotech G1 Therapeutics. Despite the emergence of targeted cancer drugs and immuno-oncology agents, chemo remains a cornerstone of treatment, but one which takes a heavy toll on patients.
Pharmacy Times
JULY 19, 2022
Logan Franck, PharmD, BCACP, a clinical associate professor in the College of Pharmacy at the University of Nebraska Medical Center, discuses his American Association of Pharmacy Technicians session on over-the-counter medications.
Pharma Times
JULY 19, 2022
Imvanex is a non-replicating smallpox vaccine developed in collaboration with the US government

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.

Board Vitals - Pharmacist
JULY 19, 2022
One of the best and, sometimes, most confusing characteristics of becoming a nurse practitioner (NP) is the ability to work in many types of jobs. However, knowing where you want to work after school can help you to distinguish what type of NP you want to become. Acute care nurse practitioners (ACNP) can work in a wide array of areas, which are typically related to short-term, serious incidences of illness.

Compounding Pharmacy of America
JULY 19, 2022
A good night’s sleep can often feel like a luxury hard to come by. Work, kids, social pressures/obligations, as well as other distractions such as technology may keep you awake far longer than they should, making you sacrifice something that is detrimental to the overall health of your body: sleep. Effects of Sleep Deprivation The […]. The post Sunlight and Sleep: A Natural Remedy to Sleep Better appeared first on The Compounding Pharmacy of America.

The Luxe Pharmacist
JULY 19, 2022
I’ve gotten so many requests for make up tutorials and what products I use on repeat, and while I don’t have a tutorial up yet this is the next best thing! I absolutely love having glowing and bright skin especially in the summer which means I’ve learned to not be afraid of my two best friends…blush and highlighter. I’ve been wearing the below combo all summer long on repeat.
Outsourcing Pharma
JULY 19, 2022
The Foundation for Sarcoidosis Research hosted more than 50 agency leaders in a session that addressed patient concerns in research, care, and diagnosis.

Advertisement
Are you still using workarounds to manage your daily operations? To achieve peak performance, it's time to explore other options for specialty and infusion pharmacy software. Streamline pharmacy operations and improve clinical performance with automated processing, real-time data exchange, and electronic decision support. Download this helpful infographic to: Drive efficiency and patient adherence from referral receipt to delivery and ongoing care – all with our Pharmacy Cloud.
Pharmaceutical Technology
JULY 19, 2022
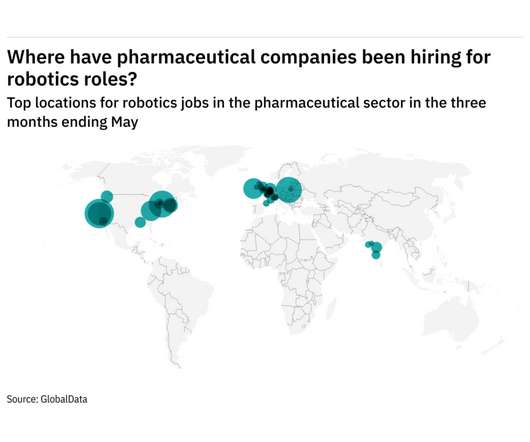
Asia-Pacific was the fastest growing region for robotics hiring among pharmaceutical industry companies in the three months ending May. The number of roles in Asia-Pacific made up 2.6% of total robotics jobs - up from 0.7% in the same quarter last year. That was followed by Europe, which saw a 0.1 year-on-year percentage point change in robotics roles.

Outsourcing Pharma
JULY 19, 2022
The CRO and medical genetics company will collaborate to accelerate trials centered on rare diseases, using real-world data and genetic testing technology.

pharmaphorum
JULY 19, 2022
AstraZeneca and Merck’s PARP inhibitor Lynparza has carved out blockbuster revenues across uses in breast, ovarian, prostate and pancreatic cancer, but colorectal cancer won’t be added to the list. Merck said the LYNK-003 rial of Lynparza (olaparib) given as either a monotherapy or in combination with Roche’s CD20-targeting antibody Avastin (bevacizumab) in advanced colorectal cancer has been halted, as a look at the unblinded data showed little chance of a positive result.

Pharmaceutical Commerce
JULY 19, 2022
Three levels of service—Silver, Gold and Platinum—are rolling out globally.

PharmExec
JULY 19, 2022
Carolyn O’Neill, Chief Creative Officer of Centron discusses their APEX-award winning global disease awareness campaign for both HCPs and patients, “Dimensionalizing the Patient Experience in PNH.” PNH or paroxysmal nocturnal hemoglobinuria is a rare immune disorder that attacks a person’s red blood cells. The campaign was meant to build on it is not an inherited disease and people’s symptoms don’t exhibit externally, usually appearing in mid-life and portray the feelings of a PNH sufferer.

Pharmaceutical Technology
JULY 19, 2022
AbbVie has filed a Marketing Authorization Application (MAA) with the European Medicines Agency (EMA) for its atogepant for prophylaxis of migraine in adults who experience a minimum of four migraine days each month. An oral CGRP receptor antagonist (gepant), atogepant is developed as a preventive migraine treatment. The application is based on the Phase III ADVANCE and PROGRESS clinical trial analysing the safety, efficacy and tolerability of atogepant in adult patients with episodic migraine a

Pharma Times
JULY 19, 2022
Specifically engineered antibody treats HER2-positive metastatic breast cancer

Pharmaceutical Commerce
JULY 19, 2022
undefined.

PharmExec
JULY 19, 2022
The move makes FINN one of the world's largest agencies in health innovation and Increases SPAG global service capabilities and offerings for clients.

pharmaphorum
JULY 19, 2022
Novartis said this morning that it remains on course to make a decision on the future of its generics business Sandoz by the end of the year, amid signs of a “return towards normal business dynamics” in the first half. The Swiss pharma group said last October that it was looking into the future of Sandoz, which could include a range of options, including retaining, spinning off and selling the business.

PharmExec
JULY 19, 2022
Award-winning healthcare, digital health and life sciences PR agency and care orchestration leader partner to raise awareness of automating manual tasks and empowering care teams.
Pharmacy Times
JULY 19, 2022
Results are first to show causal relationship between losing mLOY in the blood and development of cardiovascular disease.

PharmExec
JULY 19, 2022
Aimed at small and emerging biopharma/biotech manufacturers, this service fills a gap in the market.

European Pharmaceutical Review
JULY 19, 2022
Transpire Bio and Recipharm have signed a definitive agreement for the development of TRB-1 and TRB-2 – two inhaled medicines for the treatment of asthma and Chronic Obstructive Pulmonary Disease (COPD). TRB-1 and TRB-2 are the first products developed by Transpire Bio and are intended for advanced markets. “Our mission is to improve access to important, life-saving inhaled therapies, and to introduce new inhaled therapies to help address areas of significant unmet medical need,” stated Dr Xian-
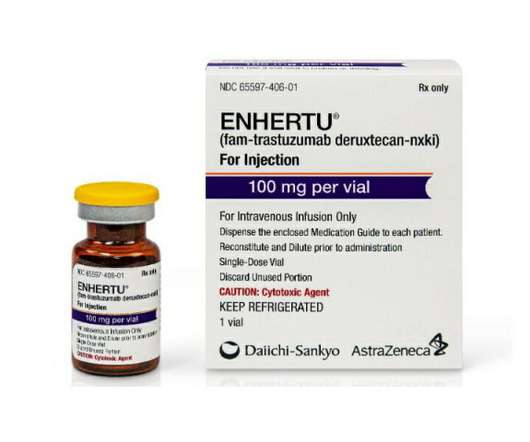
pharmaphorum
JULY 19, 2022
AstraZeneca and Daiichi Sankyo have claimed approval in the EU for Enhertu as a second-line therapy for HER2-positive metastatic breast cancer, moving the drug up the treatment pathway. The green light from the European Commission means that Enhertu (trastuzumab) deruxtecan) can now be used for patients whose disease has progressed after first-line therapies such as Roche’s Herceptin (trastuzumab) plus chemotherapy, a standard regimen for this type of cancer.
Let's personalize your content