Apple adds medication tracking to iPhone and Apple Watch
pharmaphorum
JUNE 8, 2022
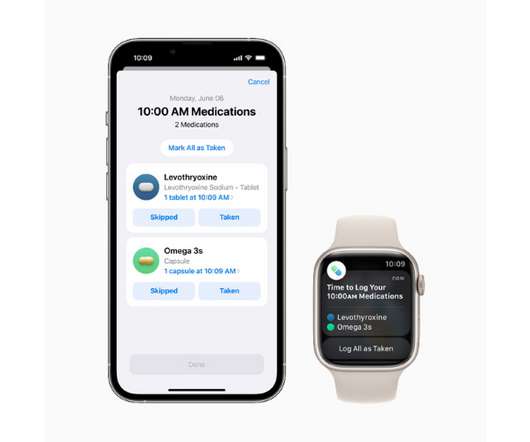
Owners of an iPhone or Apple Watch have a new health feature, an app called Medications, that will help them manage and track their use of medicines. The new tool works as a component of Apple’s Health app and will let users add drugs or other health products like vitamins and supplements to a personal list – either by scanning a label or finding the product in a directory – and create custom schedules for them.























Let's personalize your content