Smart Injector Insulin Pen Device, Mallya, Cleared by FDA
Drug Topics
JANUARY 25, 2023
Mallya can provide convenience for insulin pen users; however, it has yet to be released in the United States.

Drug Topics
JANUARY 25, 2023
Mallya can provide convenience for insulin pen users; however, it has yet to be released in the United States.

Fierce Pharma
JANUARY 25, 2023
Merck's Keytruda dealt another blow in prostate cancer but readies new challenge to AZ's Imfinzi aliu Wed, 01/25/2023 - 10:17
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Fierce Healthcare
JANUARY 25, 2023
Wave of leading medical schools pull out of U.S.

European Pharmaceutical Review
JANUARY 25, 2023
The first major study on the efficacy of DMT in treating any mental health condition showed a remission rate of 57 percent following a single dose, in a Phase IIa trial for major depressive disorder (MDD). “SPL026 (intravenous DMT) with supportive therapy was shown to have a significant antidepressant effect that was rapid and durable, with a remission rate of 57 percent at three months following a single dose of SPL026,” stated Dr Carol Routledge, Chief Medical and Scientific Officer at Small P

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Pharmacy Is Right For Me
JANUARY 25, 2023
There are so many different types of careers within the field of pharmacy—from research and drug development to pharmacy informatics! To highlight some of the more unique career settings in the industry, we’re introducing a new page on our website— Novel Pharmacy Practice Settings —where you can explore these unique career pathways. In addition to learning more about unique pathways on our new webpage, we’ll also be featuring pharmacists who work in these unique settings on our blog.

PharmaVoice
JANUARY 25, 2023
As Calcium’s medical director, Lazar is leveraging next-generation innovation to create strategic connections between science and marketing for healthcare brands.
Pharmacy Technician Pulse brings together the best content for pharmacy technicians from the widest variety of industry thought leaders.

PharmaVoice
JANUARY 25, 2023
The pharma giant's CEO Joaquin Duato and CFO Joseph Wolk showed that they're optimistic about reaching lofty goals despite hard economic times.

Fierce Healthcare
JANUARY 25, 2023
Medicare Advantage plans brace for final controversial risk adjustment rule by Feb.

Drug Store News
JANUARY 25, 2023
Kroger Health has established a clinical trial site network to increase reach and access to research studies.

Fierce Healthcare
JANUARY 25, 2023
Payer startup Angle Health scores $58M to scale operations, expand to new markets agliadkovskaya Wed, 01/25/2023 - 09:42

Speaker: Chris Antypas and Josh Halladay
Access to limited distribution drugs and payer contracts are key to pharmacy expansion. But how do you prepare your operations to take the next step? Meaningful data: Collect and share clinical data regarding outcomes, utilization, and more Reporting: Limited distribution models require efficient tracking and reporting systems Workflows: Align workflows with specific pharma and payer contractual requirements For in-depth, expert insights on pharmacy expansion, watch this webinar from Inovalon.

European Pharmaceutical Review
JANUARY 25, 2023
The European Medicines Agency (EMA) has validated the Marketing Authorization Application (MAA) for exagamglogene autotemcel (exa-cel), the first regulatory submission for a CRISPR-based medicine. Through the validation, exa-cel is indicated for the treatment of sickle cell disease (SCD) and transfusion-dependent beta thalassemia (TDT). The MAA from Vertex Pharmaceuticals and CRISPR Therapeutics is supported by two global Phase III studies.

Fierce Healthcare
JANUARY 25, 2023
State-by-state licensure is anachronistic in the age of teletherapy, providers say aburky Wed, 01/25/2023 - 14:52

Fierce Pharma
JANUARY 25, 2023
After years of back-and-forth, Gilead's CAR-T Yescarta sways England's cost watchdog NICE fkansteiner Wed, 01/25/2023 - 18:51

Fierce Healthcare
JANUARY 25, 2023
Health system hospitals, physicians deliver 'marginally' better care at higher cost than independent peers: study dmuoio Wed, 01/25/2023 - 13:52

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.

pharmaphorum
JANUARY 25, 2023
Digital therapeutics company Woebot Health has announced enrolment of the first patient in a pivotal clinical trial to evaluate the safety and efficacy of WB001, an investigational therapeutic for postpartum depression (PPD), granted Breakthrough Device designation by the US Food and Drug Administration (FDA) in 2021. The double-blind, randomised, controlled trial will evaluate WB001, a Software as a Medical Device (SaMD) based Cognitive Behavioural Therapy (CBT) program for the treatment of mil

Fierce Pharma
JANUARY 25, 2023
As Scangos bids adieu, Vir taps Bayer dealmaker De Backer as its next CEO fkansteiner Wed, 01/25/2023 - 11:09

STAT
JANUARY 25, 2023
Three years ago, the World Health Organization declared that the mushrooming outbreak of a new coronavirus — later named SARS-CoV-2, the cause of Covid-19 — posed such a threat to global health that it merited designation as a public health emergency of international concern. On Friday, an emergency committee will meet again to deliberate whether the time has come to recommend to WHO Director-General Tedros Adhanom Ghebreyesus that he declare the global health emergency is over.

Med Ed 101
JANUARY 25, 2023
In this article, we lay out our top 5 all-time hypertension clinical trials. If you remember previously, we covered the top 5 landmark clinical trials in hyperlipidemia. It was a tough call with many options, but here’s our list of hypertension clinical trials. Feel free to comment below if you feel we missed one! DASH […] The post Top 5 All-Time Hypertension Clinical Trials appeared first on Med Ed 101.

Advertisement
Are you still using workarounds to manage your daily operations? To achieve peak performance, it's time to explore other options for specialty and infusion pharmacy software. Streamline pharmacy operations and improve clinical performance with automated processing, real-time data exchange, and electronic decision support. Download this helpful infographic to: Drive efficiency and patient adherence from referral receipt to delivery and ongoing care – all with our Pharmacy Cloud.

Drug Store News
JANUARY 25, 2023
This year’s winners pay homage to legacy brands that have been mainstays in the drug store channel’s natural section as well as new brands that have created a name for themselves.

STAT
JANUARY 25, 2023
After a year defined by record inflation and double-digit health care premium increases, I hope that a few years down the road we can eventually look back at 2023 as the year that ignited change in U.S. health care and led to a new system that future generations are proud of. I’ll be doing everything I can to make that happen.

Drug Store News
JANUARY 25, 2023
H-E-B officially opened its store in Cibolo, Texas, the first location for the retailer in the community northeast of San Antonio.

pharmaphorum
JANUARY 25, 2023
Life sciences marketing has rapidly evolved to mirror that of consumer retail. There are now more ways than ever to interact with customers, from traditional channels like email and phone calls to more modern approaches like social media and texting, and new ways to engage customers are emerging every year. So, how can companies maximise the effectiveness of customer engagement strategies when there are so many digital and physical mediums of interaction to choose from?

Drug Store News
JANUARY 25, 2023
Walmart announced four initiatives to support its associates, including offering higher wages.
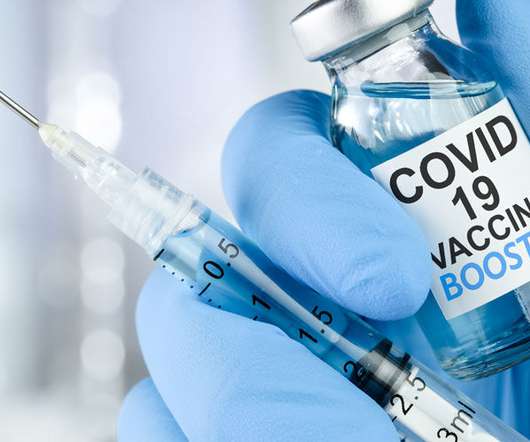
Hospital Pharmacy Europe
JANUARY 25, 2023
The Joint Committee on Vaccination and Immunisation (JCVI) has advised offering Covid booster vaccinations in spring and autumn 2023 for patients deemed to be at high risk. Interim advice to the UK Government has recommended that planning begin for a 2023 autumn booster programme for those at higher risk of severe Covid-19 because of age or clinical risk factors.

Drug Store News
JANUARY 25, 2023
The virtual care program is now available nationwide to Aetna commercial members enrolled in eligible fully insured and self-insured health plans.

pharmaphorum
JANUARY 25, 2023
Following the European Society of Gynaecological Oncology (ESGO) 2022 congress, pharmaphorum spoke with experts from among the Ovarian Cancer Commitment (OCC) founding partners – ESGO, the European Network of Gynaecological Cancer Advocacy Groups (ENGAGe), and AstraZeneca – all of which had been involved in the development of Olivia: a new digital and dedicated support resource for people affected by ovarian cancer through all stages of the disease.

STAT
JANUARY 25, 2023
Vir Biotechnology CEO George Scangos said on Wednesday he will retire, ending an itinerant, nearly 40-year-career as one of biotech’s most recognizable executives. Scangos, 74, will be succeeded in April by Marianne De Backer , a longtime pharma executive who currently heads pharmaceutical strategy at Bayer. She will inherit a company that rose to prominence during the pandemic by developing the antibody drug sotrovimab and is trying to repeat the success with a series of candidates for C

The Checkup by Singlecare
JANUARY 25, 2023
If you’ve decided it’s time to lose the extra weight you’ve been carrying around, here’s some motivation: You can experience a number of health benefits from just a modest amount of weight loss. Losing just 5% to 10% of your total body weight can help you sleep better at night, maintain better control over your blood sugar levels, and lower your risk of cardiovascular disease, according to the Centers for Disease Control and Prevention (CDC).

STAT
JANUARY 25, 2023
In a rare move, the Better Business Bureau National Programs settled a dispute between two big drug companies over a prescription drug ad, suggesting the nonprofit may take a larger role in resolving complaints that might normally be handled by the U.S. Food and Drug Administration. The dispute began when Eli Lilly challenged some of the information in an ad that Novartis had run for its Kisqali breast cancer treatment.

Drug Store News
JANUARY 25, 2023
Drug Store News spoke to Howard Murad, a board-certified dermatologist, pharmacist and founder of Murad Skincare, about the brand’s new Eczema Control line and how it caters to all consumers.

Hospital Pharmacy Europe
JANUARY 25, 2023
Glyphosate is commonly used as a herbicide and a study in farmers has observed positive associations with biomarkers of oxidative DNA damage Regular use by farmers of the herbicide glyphosate is linked with higher levels of urinary biomarkers of oxidative stress and which represents a key characteristic of carcinogens, according to a study by US researchers.

Drug Store News
JANUARY 25, 2023
The agency told its staff that it expects to make several changes internally, with some offices merging their responsibilities and new offices being created, according to a report.

STAT
JANUARY 25, 2023
Almost a year ago, three top investors at Lux Capital and Obvious Ventures announced they were leaving the firms, teasing on Twitter that they were starting “something new.” That something is a new venture capital firm, which launched Wednesday with $350 million for its first fund.
Let's personalize your content