First 90 Days: Anjarium Biosciences' Stephen Yoo
PharmaVoice
JULY 15, 2022
The incoming CEO aims to build on the buzz around the company’s leading-edge gene therapy tech.

PharmaVoice
JULY 15, 2022
The incoming CEO aims to build on the buzz around the company’s leading-edge gene therapy tech.

Pharmaceutical Technology
JULY 15, 2022
Insulin prices made the headlines again as California governor Gavin Newsom announced plans on 7 July for the state to manufacture low-cost insulin. In a budget change proposed in February and confirmed in May , California’s Department of Health Care Access and Information (HCAI) requested a one-time investment of $100 million for Newsom’s CalRx Biosimilar Insulin initiative.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

pharmaphorum
JULY 15, 2022
Akili looks like it could bring another digital therapeutic (DTx) to market after a clinical trial backed the efficacy of its AKL-T01 in patients with the autoimmune disorder systemic lupus erythematosus (SLE). The PureTech group company – which scored the first FDA and EMA approvals for a DTx for attention-deficit hyperactivity disorder (ADHD) – is developing AKL-T01 to tackle cognitive impairments that can affect people with SLE.

Pharmacy Times
JULY 15, 2022
The FDA approved tirzepatide for the treatment of type 2 diabetes on May 13, 2022.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

pharmaphorum
JULY 15, 2022
Pfizer’s tyrosine kinase inhibitor Xalkori has picked up a fourth approval from the FDA, adding a new use in the treatment of a rare form of benign tumour that typically affects children and young adults. Xalkori (crizotinib) has been cleared for inflammatory myofibroblastic tumours (IMT), which appear in organs like the lung, stomach, bladder or liver.

Pharmacy Times
JULY 15, 2022
Triptans are contraindicated in patients with ischemic heart disease, cerebrovascular disease, uncontrolled hypertension, and peripheral artery disease.
Pharmacy Technician Pulse brings together the best content for pharmacy technicians from the widest variety of industry thought leaders.

Pharmacy Times
JULY 15, 2022
Ryan Burke, PharmD, director of professional affairs at Pharmacy Technician Certification Board, discusses more advanced roles for pharmacy technicians and how they can continue to grow beyond their current roles.
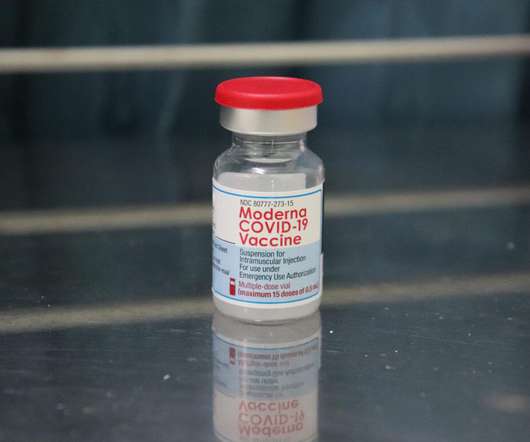
Pharmaceutical Technology
JULY 15, 2022
Health Canada has granted approval for the usage of Moderna’s messenger RNA (mRNA) Covid-19 vaccine, Spikevax, in a 25µg two-dose regimen for active immunisation to prevent Covid-19 in children aged six months to five years. So far, children aged below five years were not eligible to receive the Covid-19 vaccine in Canada. The two-dose initial vaccine regimen in children of this age group is completed in one month, which is the same dosing schedule in adults, adolescents and children aged above

Pharmacy Times
JULY 15, 2022
Debbie Weitzman of Cardinal Health, discussed how independent retail pharmacies quickly adjusted during the pandemic.

European Pharmaceutical Review
JULY 15, 2022
The pharmaceutical sector is not typically seen as a highly polluting, ‘heavy industry’ but it is far from green. In its 2021 report Delivering a ‘Net Zero’ National Health Service , the UK’s NHS attributes as much as a quarter of its carbon footprint to medicines. A deep carbon footprint is a common hallmark of energy intensive manufacturing processes – and the manufacture of pharmaceuticals is no exception.

Speaker: Chris Antypas and Josh Halladay
Access to limited distribution drugs and payer contracts are key to pharmacy expansion. But how do you prepare your operations to take the next step? Meaningful data: Collect and share clinical data regarding outcomes, utilization, and more Reporting: Limited distribution models require efficient tracking and reporting systems Workflows: Align workflows with specific pharma and payer contractual requirements For in-depth, expert insights on pharmacy expansion, watch this webinar from Inovalon.

PharmExec
JULY 15, 2022
Podcast recognized for its excellence in apps and podcasts category.
pharmaphorum
JULY 15, 2022
Health technology assessment (HTA) agency NICE has finalised its guidance on Amarin’s Vazkepa, clearing the path for GPs to start prescribing the drug in up to 425,000 NHS patients at high cardiovascular risk because of raised triglyceride levels. The just-published final technology appraisal document recommends that Vazkepa (icosapent ethyl) can be prescribed to people in England and Wales for adult patients with high-risk cardiovascular disease and elevated levels of triglycerides (1.7 m

European Pharmaceutical Review
JULY 15, 2022
A report assessing the current climate for antimicrobial innovation in Brazil, India, and South Africa, reveals that removing regulatory hurdles to accelerate the approval of new antibiotics will help to improve their development and access. Antimicrobial resistance (AMR) is a growing challenge to the healthcare sector, as antibiotics are a linchpin of modern medicine.
Pharmacy Times
JULY 15, 2022
Pharmacists can recommend calcium, smoking cessation products, and vitamin D to stave off this common condition.

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.

PharmExec
JULY 15, 2022
Industry leaders discuss new technologies, research, and investment strategies.

Pharmacy Times
JULY 15, 2022
Desitin is used to provide relief of skin irritation caused by a diaper rash.
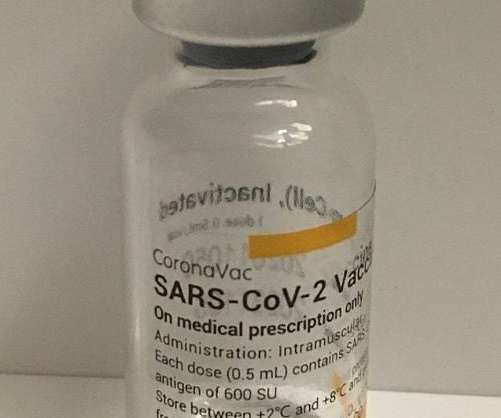
Pharmaceutical Technology
JULY 15, 2022
The Brazilian Health Regulatory Agency (Anvisa) has granted emergency use authorization for Sinovac Biotech’s Covid-19 vaccine, CoronaVac, in children aged three to five years. Subjects in this age group can receive the same vaccine dosage authorised in patients aged six to 17 years, as well as adults. Furthermore, there exists no restriction on vaccine usage in immunosuppressed children aged three to five years.
Pharmacy Times
JULY 15, 2022
Oritavancin (Orbactiv) is indicated for the treatment of adult patients with acute bacterial skin and skin structure infections caused or suspected to be caused by susceptible isolates of designated Gram-positive microorganisms.

Advertisement
Are you still using workarounds to manage your daily operations? To achieve peak performance, it's time to explore other options for specialty and infusion pharmacy software. Streamline pharmacy operations and improve clinical performance with automated processing, real-time data exchange, and electronic decision support. Download this helpful infographic to: Drive efficiency and patient adherence from referral receipt to delivery and ongoing care – all with our Pharmacy Cloud.

Pharma Times
JULY 15, 2022
Data from APHINITY study shows Roche’s Perjeta-based regimen reduces the risk of disease returning for people with HER2-positive early breast cancer

Pharmacy Times
JULY 15, 2022
Pursuing the expanded access process for treatment may be the best option for patients who have exhausted all available FDA-approved drugs and clinical trials.

Pharmaceutical Commerce
JULY 15, 2022
Partnership allows for distribution of packager’s temperature-controlled container.

Pharmacy Times
JULY 15, 2022
Provepharm filed its application with the agency in September 2021, and, with the new approval, its US affiliate will market the product directly in the United States.

PharmExec
JULY 15, 2022
Patients with Crohn's disease and ulcerative colitis face a high disease burden.

Pharmacy Times
JULY 15, 2022
Investigators argue that much of the language around women who give birth later in life is rooted in ableism and ageism and is out of step with current childbirth trends.

Outsourcing Pharma
JULY 15, 2022
Clinical Trial Navigator, from AmerisourceBergen, is designed to help sponsors pair with oncology practices to boost patient recruitment opportunities.

Pharmacy Times
JULY 15, 2022
Being able to acknowledge those missteps that may have been made that may have prevented you from delivering as culturally competent care, and being able to recalibrate and to take the correction is an important step.

pharmaphorum
JULY 15, 2022
SAE Media Group’s 5th Annual. Ophthalmic Drugs Conference. 21 – 22 November 2022 | London, UK. [link]. Delving into advanced R&D and Delivery of Ocular Therapeutics. SAE Media Group’s 5th Annual Ophthalmic Drugs Conference will explore advancements in ophthalmic treatments and variety of novel drugs, which are showing good clinical trial data.

Pharmacy Times
JULY 15, 2022
Crizotinib has also been approved to treat non-small cell lung cancer that has spread to other parts of the body and is caused by a defect in either the ALK or ROS1 gene.

pharmaphorum
JULY 15, 2022
Teva has asked the Supreme Court to look at a judgment in a $235 million patent dispute with GSK that it claims could undermine the ability of generic drugmakers to bring new products to market, and thereby help reduce drug prices. The case hinges around the concept of “ skinny labelling ” which was introduced to stop drugmakers extending the exclusivity period for their branded medicines – through a stream of new indications or patient populations – that can be protected with so-cal

Pharmacy Times
JULY 15, 2022
Pharmacies have a lot of opportunities to think out of the box, to be innovative, and continue to drive that power of community that we're highlighting at the event here.

Fossil Remedies
JULY 15, 2022
Cardiac and Diabetic. In recent years, diabetes and heart diseases are quite common. They are called lifestyle diseases because the fundamental cause is sedentary life and lack of exercise. As the number of people affected by these problems is increasing, there is a high demand for medicines required to treat them. If you are planning to launch Cardiac Diabetic PCD Pharma Franchise, then it is the best time.

pharmaphorum
JULY 15, 2022
Illumina’s attempt to prevent the European Commission from carrying out an antitrust review of its $8 billion takeover last year of cancer diagnosis firm Grail has been defeated in an EU court. The EU General Court has ruled that the Commission can investigate the merger, unswayed by Illumina’s argument that it should have no jurisdiction over the deal because Grail does not currently have a commercial presence within the EU.

Pharma Times
JULY 15, 2022
Armed forces veterans and people who were exposed to asbestos could benefit from the therapy
Let's personalize your content