Pharmacist-Prescribed Paxlovid for COVID-19 Now Available at CVS
Drug Topics
NOVEMBER 18, 2022
CVS is the first pharmacy to offer this service nationwide.

Drug Topics
NOVEMBER 18, 2022
CVS is the first pharmacy to offer this service nationwide.

PharmaVoice
NOVEMBER 18, 2022
Executives across the life sciences share their tips for navigating the new norm of remote work.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Drug Topics
NOVEMBER 18, 2022
Jay Dombi and Lamont Robinson share how the AmerisourceBergen Marketplace can help pharmacies and why having diverse suppliers is important.

Fierce Healthcare
NOVEMBER 18, 2022
From finding the right candidates to keeping them, how hospitals are using AI to address workforce needs. aburky. Fri, 11/18/2022 - 15:25.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Drug Topics
NOVEMBER 18, 2022
Your weekly roundup of the latest news from Drug Topics®.

STAT
NOVEMBER 18, 2022
A federal judge on Friday sentenced disgraced Theranos CEO Elizabeth Holmes to more than 11 years in prison for duping investors in the failed startup that promised to revolutionize blood testing but instead made her a symbol of Silicon Valley’s culture of audacious self-promotion. The sentence imposed by U.S. District Judge Edward Davila was shorter than the 15-year penalty requested by federal prosecutors but far tougher than the leniency her legal team sought for the mother of a year-o
Pharmacy Technician Pulse brings together the best content for pharmacy technicians from the widest variety of industry thought leaders.
Pharmacy Times
NOVEMBER 18, 2022
Teplizumab-mzwv is administered through injection and can delay the onset of type 1 diabetes in adults and pediatric patients.

Drug Topics
NOVEMBER 18, 2022
Expert panelists highlight special considerations when selecting COVID-19 vaccines.

Pharmacy Times
NOVEMBER 18, 2022
Education and support inspire trust between patients and pharmacists.
Drug Topics
NOVEMBER 18, 2022
With closing remarks, Mitchel Rothholz, RPh, MBA, and Jeff Goad, PharmD, MPH, discuss common myths circulating around COVID-19 vaccines and vaccination.

Speaker: Chris Antypas and Josh Halladay
Access to limited distribution drugs and payer contracts are key to pharmacy expansion. But how do you prepare your operations to take the next step? Meaningful data: Collect and share clinical data regarding outcomes, utilization, and more Reporting: Limited distribution models require efficient tracking and reporting systems Workflows: Align workflows with specific pharma and payer contractual requirements For in-depth, expert insights on pharmacy expansion, watch this webinar from Inovalon.

European Pharmaceutical Review
NOVEMBER 18, 2022
Upstaza (eladocagene exuparvovec) is the first and only approved treatment for aromatic L-amino acid decarboxylase (AADC) deficiency and is the first marketed gene therapy for direct infusion into the brain. The product, produced by biopharma company PTC Therapeutics, is approved for patients 18 months and over. It has been granted marketing authorisation by the Medicines and Healthcare Products Regulatory Agency (MHRA) in Great Britain.
Pharmacy Times
NOVEMBER 18, 2022
Patients administered 75 mg or higher of olpasiran every 12 weeks had a 95% or greater reduction in Lp(a) compared to placebo at week 36.

Fierce Healthcare
NOVEMBER 18, 2022
CDC: Home births soared to a 30-year high in 2021. fdiamond. Fri, 11/18/2022 - 14:51.
Pharmacy Times
NOVEMBER 18, 2022
Dapagliflozin is the first SGLT2 inhibitor approved by the FDA for treatment of patients with heart failure (NYHA class II-IV) with reduced ejection fraction.

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.

STAT
NOVEMBER 18, 2022
BOSTON — As Pfizer prepares to hike the price of its Covid-19 vaccines, the company’s CEO, Albert Bourla, maintained at a conference this week that the jabs will continue to be “free for all Americans” because insurers are required to pay the extra cost. “Americans will see no difference,” said Bourla, speaking Wednesday at the STAT Summit.

Pharmacy Times
NOVEMBER 18, 2022
Annie Lambert, PharmD, BCSCP, discusses how to approach the implementation of the revised USP chapter guidelines for , , and before the enforcement date in fall 2023.

STAT
NOVEMBER 18, 2022
Biosplice’s bid to transform the treatment of everything from arthritis to cancer ran into a snag this week, with the San Diego biotech announcing that its experimental osteoarthritis drug failed to benefit patients in a pair of Phase 3 clinical trials. The results, presented at the American College of Rheumatology conference in Philadelphia, show that the small-molecule drug lorecivivint failed to significantly soothe the pain of osteoarthritis patients.

Pharmacy Times
NOVEMBER 18, 2022
A compliance officer role represents an exciting new challenge to ensure safe handling in compliance with United States Pharmacopeia.

Advertisement
Are you still using workarounds to manage your daily operations? To achieve peak performance, it's time to explore other options for specialty and infusion pharmacy software. Streamline pharmacy operations and improve clinical performance with automated processing, real-time data exchange, and electronic decision support. Download this helpful infographic to: Drive efficiency and patient adherence from referral receipt to delivery and ongoing care – all with our Pharmacy Cloud.

Fierce Healthcare
NOVEMBER 18, 2022
Sanders, Cassidy in line to lead pivotal Senate HELP committee in next Congress. rking. Fri, 11/18/2022 - 16:16.
pharmaphorum
NOVEMBER 18, 2022
Regeneron Pharmaceuticals, Inc. and CytomX Therapeutics, Inc. have announced a strategic research collaboration within the field of conditionally activated bispecific therapeutics for the treatment of cancer. Using CytomX’s Probody and Regeneron’s Veloci-Bi platforms, the collaboration and licensing agreement aims to enable the development of investigational next-generation bispecific immunotherapies.

STAT
NOVEMBER 18, 2022
Nectar bats may have nature’s biggest sweet tooth. Each night, the creatures scour the jungle for blossoming flowers in order to consume as much as 150% of their body weight in liquid sugar. Such a feat would leave other mammals in shock or in a coma. But nectar bats push biology to the limit — making them “wonderful, majestic flying superheroes,” in the words of Jasmin Camacho, a postdoctoral researcher at the Stowers Institute for Medical Research who was recently n

The Thyroid Pharmacist
NOVEMBER 18, 2022
Thanksgiving is my favorite American holiday. It’s a day to count our blessings and be thankful for all of the goodness we have in our lives… especially given the challenges we’ve faced over the past few years. Thanksgiving has always been a day to be with family and loved ones, to prepare and enjoy beautiful food together. But like the last two years, some of us may be celebrating Thanksgiving 2022 alone or with smaller groups than we’re used to.
Pharmaceutical Technology
NOVEMBER 18, 2022
The US Food and Drug Administration (FDA) has approved Provention Bio’s biologics licence application (BLA) for Tzield (teplizumab-mzwv) to treat type 1 diabetes (T1D) patients. The anti-CD3-directed antibody Tzield aims to delay the onset of Stage 3 T1D in adults and children aged eight years and above who are currently with stage 2 T1D. It is claimed to be the first and only immunomodulatory treatment for T1D and is provided as a sterile, preservative-free, clear, and colourless solution in a

STAT
NOVEMBER 18, 2022
Amid ongoing concerns over the use of medically important antibiotics given to food-producing livestock and farmed fish, sales of veterinary medicines across Europe dropped by nearly half between 2011 and 2021, which regulators reported is the biggest drop ever recorded and a sign that campaigns to reduce use are working. At issue is the extent to which antibiotics are given to food-producing livestock to prevent and treat disease — a practice that has contributed to an alarming rise in a

European Pharmaceutical Review
NOVEMBER 18, 2022
Tzield (teplizumab-mzwv), the first drug to help prolong the onset of stage 3 type 1 diabetes in adults and children over eight years old with stage 2 type 1 diabetes, has been approved by the US Food and Drug Administration (FDA). “Today’s approval of a first-in-class therapy adds an important new treatment option for certain at-risk patients,” remarked Dr John Sharretts, director of the Division of Diabetes, Lipid Disorders, and Obesity in the FDA’s Center for Drug Evaluation and Research.
STAT
NOVEMBER 18, 2022
Biosimilars, a much-lauded approach to reducing drug costs in the United States, are still underused here, even as they are proving successful in Europe. Why? Two key reasons are misperceptions of inferiority and the intricacies of U.S. market access. As the head of U.S. market access for Samsung Bioepis, a South Korean developer and manufacturer of biosimilars, I routinely introduce our capabilities to key stakeholders in the U.S. health care market.
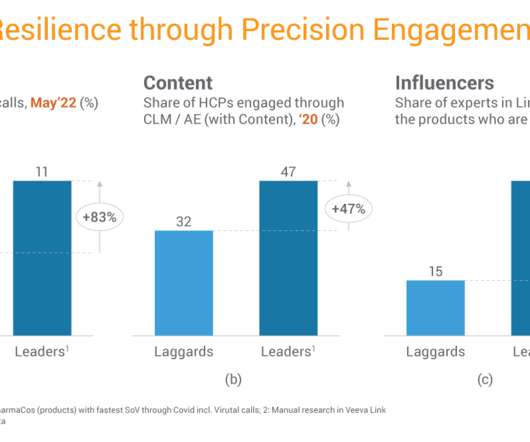
pharmaphorum
NOVEMBER 18, 2022
Karl Goossens, director of commercial analytics at Veeva Systems Europe, recently spoke with pharmaphorum, discussing how effective omnichannel execution drives revenue and productivity. Biopharma’s digital interactions with healthcare professionals (HCPs) are now a critical part of industry relationships. Even with the era of widespread COVID-19 lockdowns behind us, today’s macroeconomic uncertainty and growing inflation make it more critical for commercial organisations to continue their digit

European Pharmaceutical Review
NOVEMBER 18, 2022
Advanced BioDesign , a French biotechnology company specialising in the development of targeted therapies in oncology, announced that the first patient has been treated, at the Centre d’Investigation des Thérapeutiques en Oncologie et Hematologie de Lyon (CITHOL), with its first-in-class drug candidate: ABD-3001 for acute myeloid leukaemia. . ABD-3001 is the pharmaceutical form of DIMATE, which targets and inhibits a detoxification system present in cancer cells.

pharmaphorum
NOVEMBER 18, 2022
Takeda has a chance of breaking into new territory with its blood cancer therapy Iclusig, after the drug performed better than mainstay therapy imatinib in a phase 3 study of adults with newly-diagnosed Philadelphia chromosome-positive acute lymphoblastic leukaemia (Ph+ ALL). The PhALLCON trial compared Iclusig (ponatinib) to imatinib – sold by Novartis as Glivec/Gleevec, but also available as a generic – on top of a reduced-intensity chemotherapy regimen in 230 patients with this type of leukae

STAT
NOVEMBER 18, 2022
Hired someone new and exciting? Promoted a rising star? Finally solved that hard-to-fill spot? Share the news with us, and we’ll share it with others. That’s right. Send us your changes, and we’ll find a home for them. Don’t be shy. Everyone wants to know who is coming and going. And here is our regular feature in which we highlight a different person each week.
European Pharmaceutical Review
NOVEMBER 18, 2022
The World Health Organization (WHO)’s Global Vaccine Market Report 2022 , the first report to examine the impact of COVID-19 on the global vaccine market, shows that inequitable distribution is not unique to COVID-19 vaccines, with poorer countries consistently fighting to access vaccines in demand by wealthier countries. The WHO stated limited vaccine supply and unequal distribution is driving global inequalities.

Fierce Healthcare
NOVEMBER 18, 2022
Moving from data collection to action: Health system execs weigh in on addressing disparities systemwide. agliadkovskaya. Fri, 11/18/2022 - 17:07.
pharmaphorum
NOVEMBER 18, 2022
Gene-testing specialist Editas Medicine has halted development of its lead clinical programme for congenital eye disorders after it generated lacklustre results in a phase 1/2 trial. The BRILLIANCE study of EDIT-101 in Leber congenital amaurosis type 10 (LCA10) – an inherited form of blindness – was reported to be the first ‘in vivo’ CRISPR/Cas9 medicine to be administered to a patient when it started in 2020.
Let's personalize your content