Discover, Delve, and Develop: Expert Workshops at the 2023 ISPE Annual Meeting & Expo
ISPE
AUGUST 30, 2023
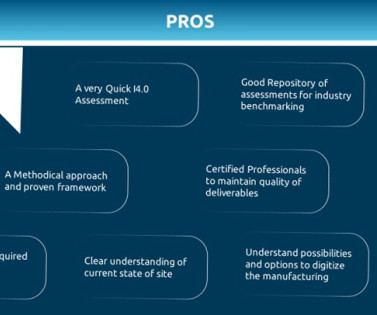
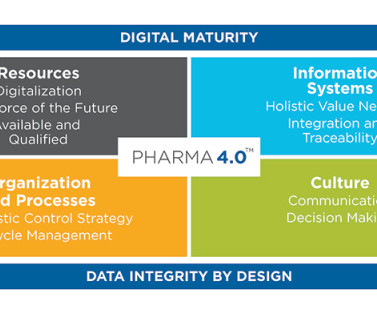
The workshops will provide students, emerging leaders, and seasoned industry professionals the opportunity to engage in thought-provoking conversations leading to the to exploration of innovative solutions to the challenges our industry faces today. Digital Transformation (DT): The ISPE Pharma 4.0™ The Pharma 4.0™ Maturity Assessment.



























Let's personalize your content