Pharmacy Leaders Enhance Culture with Continuous Learning | McKesson ideaShare
Drug Topics
JULY 26, 2025
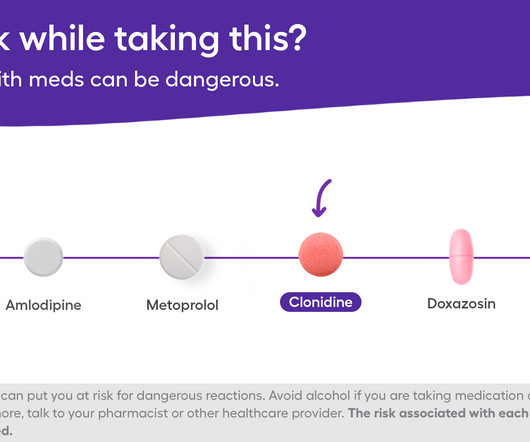
Jones advocates for continuous education, suggesting that even brief, informal learning moments can significantly enhance team knowledge and operational safety. Stay ahead of the curve with the Drug Topics newsletter and get the latest drug information, industry trends, and patient care tips. Subscribe Now!









































Let's personalize your content