FDA cracks down on off-label drug use messaging
Pharmaceutical Technology
OCTOBER 24, 2023
The FDA released a draft guidance giving firms recommendations on provider-directed communication for off-label drug use.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Pharmaceutical Technology
OCTOBER 24, 2023
The FDA released a draft guidance giving firms recommendations on provider-directed communication for off-label drug use.
Pharmaceutical Technology
MARCH 22, 2023
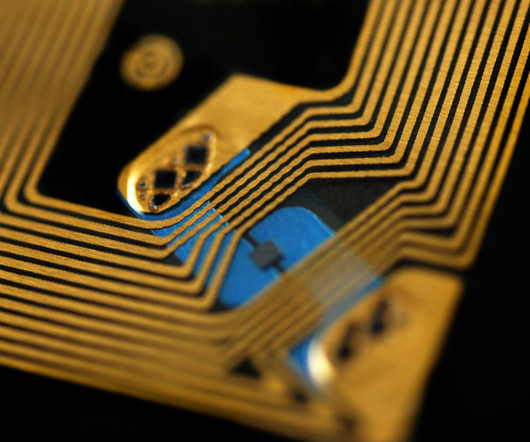
What lies ahead for RFID and smart labelling Volpe says “the future is bright” for smart technologies that identify, monitor, and track medications through the supply chain. RFID is an important facet of smart labelling and its evolution, but not the only one.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
APRIL 11, 2023
This label expansion makes Orkambi the only disease-modifying CF medication available to patients of this age in Canada. This label expansion follows a similar label change in the US in September 2022. Health Canada is granting this new label expansion based on recent results from a Phase III study.
Roots Analysis
MARCH 8, 2023
In recent years, the use of smart labels allows the developer to convey a greater amount of information about the product to the consumers, without the need for additional packaging space. Further, smart labels play a critical role in the manufacturing of pharmaceuticals.

STAT
AUGUST 20, 2024
Sentinel draws upon clinical records and insurance claims, and the agency uses its analyses to adjust drug labels, convene advisory committees and disseminate drug safety communication, the authors noted.

BuzzRx
JANUARY 27, 2023
The term “on-label use” of a drug may seem unfamiliar to most people. In short, this practice is referred to as “off-label” drug use. Surveys have shown that approximately 1 in 5 prescriptions in the US are for off-label use. In certain populations of patients, off-label drug use is even higher.

The FDA Law Blog
OCTOBER 24, 2023
By Dara Katcher Levy — Yesterday, FDA published a new Draft Guidance, “ Communications from Firms to Health Care Providers Regarding Scientific Information on Unapproved Uses of Approved/Cleared Medical Products Questions and Answers ” (SIUU Guidance or Draft Guidance).
STAT
AUGUST 25, 2023
Many see these models as assistants or even potential replacements for time-intensive tasks, like patient-physician communication through the electronic health record. As a result, they have been labeled “foundation models.

Pharma Marketing Network
APRIL 20, 2025
Introduction Is Big Pharma a villainous label or a misunderstood brand? This article explores the nuances of how this label affects pharmaceutical marketing and what brands can do to respond strategically. Be proactive in communication. This means silence or vagueness can be just as damaging as bad publicity.

The FDA Law Blog
AUGUST 23, 2022
Ironically, a recent FDA safety communication points to a potential way out of this dilemma. In this safety communication, FDA advises the public as follows: If you test negative and have COVID-19 symptoms , you test again 48 hours later for a total of two tests.

PharmExec
JULY 7, 2023
Newly-issued final guidance focuses on language used when communicating quantitative efficacy or risk information.

Digital Pharmacist
OCTOBER 11, 2023
These events can cover various topics, such as understanding prescription labels, proper medication use, managing chronic conditions, and reading nutritional labels. You can host a workshop or seminar at your pharmacy location or at a restaurant in your community.

Pharmaceutical Technology
JUNE 19, 2023
Issues with regulatory requirements and documentation can also cause significant delays, while any inaccuracies in translations on labelling can mean that dosage and storage information is not correctly understood or followed. And accurate labelling and translation are critical for this sector.

The FDA Law Blog
APRIL 25, 2024
Promotional labeling is generally any labeling other than FDA-required labeling that is devised for the promotion of a product, as well as other functions, and can include printed, audio, or visual matter that describes the product. l)(1) (e.g.,
pharmaphorum
AUGUST 24, 2020
In the United States, the 21st Century Cures Act encouraged the Food and Drug Administration (FDA) to review and communicate patient experience data from trials – but the lack of a common framework for submissions and space on product labels has, until now, been something of a stumbling block. .

Pharma Marketing Network
MARCH 27, 2025
The shift is largely due to changes in user behavior, regulatory clarity, and the demand for more personalized, real-time communication. Social is no longer one-way communication. Brands can host live Q&As, respond to comments, and share patient stories in authentic ways that create community and trust.

Pharma Marketing Network
MARCH 31, 2025
As industry leaders adapt to digital-first communication and omnichannel engagement, the need for smart, scalable, and strategic marketing tactics is more urgent than ever. Similarly, patients benefit from communications that resonate with their condition, treatment stage, and lifestyle.

pharmaphorum
OCTOBER 6, 2020
Lack of communication on how the workforce will be re-purposed post automation implementation can lead to internal unrest and possible attrition”. Label Authoring and Tracking. Lack of communication on how the workforce will be re-purposed post automation implementation can lead to internal unrest and possible attrition.
pharmaphorum
JULY 20, 2021
The US regulator had extended its review of the drug by three months – setting back its action date from April to 29 July – but now says that deficiencies in the marketing application “preclude discussion” of labelling and post-marketing requirements.

IDStewardship
APRIL 16, 2025
Each bottle was labeled with the data point and the year. BONUS POINT: This session also included a lecture by Dr. Angela Huttner, who is the current editor-in-chief of the wonderful journal Clinical Microbiology Infection (CMI) Communications. She went through some basics of writing in English.

Pharmaceutical Technology
APRIL 17, 2023
However, the treatment’s label features a black box warning that includes increased mortality in elderly patients with dementia-related psychosis treated with antipsychotic drugs. He added that there is also a need for FDA-approved products that communicate efficacy and safety on their labels.

pharmaphorum
OCTOBER 27, 2022
The overarching principle set out in Codes of Practice, and in particular the Principles for the use of digital channels in the EFPIA Code , is that the legislation and Codes of Practice apply equally to communications by companies on social media and digital channels.

Pharmaceutical Technology
JULY 20, 2022
Ganio says that, ideally, once an individual receives a positive Covid-19 result, they should call their pharmacy or communicate through a drive-through facility to minimize the risk of exposure. The US FDA has not announced any updates to Lagevrio’s label. How to get Paxlovid.

European Pharmaceutical Review
JANUARY 23, 2023
MSSG stresses the importance of transparency in relation to shortages and highlights the need for all stakeholders to communicate in an objective and responsible way to avoid any undue public concern. In addition, MSSG supports temporary national measures such as unit dose dispensing and compounding.

Hospital Pharmacy Europe
JANUARY 16, 2025
Here, lead author and focus group chair Hala Fadda discusses the reasons for widespread off-label prescribing, the FIP studys rationale and main findings, compounding challenges and considerations for improving clinical practice.

Pharmaceutical Technology
JULY 29, 2022
Dr Jeremy Veenstra-VanderWeele, professor of developmental neuropsychiatry at the Columbia University Irving Medical Center, explains that agitation is seen in the minority of autistic teens, who struggle with communication. An inability to clearly communicate with others and express their wants and needs results in frustration, he adds.

pharmaphorum
MAY 25, 2022
Where it is impossible to restrict access to HCPs, due to the congress platform or equivalent, there must be a clear statement to the attendee that the materials/communication are designed and intended for HCPs only.”. Identifying the appropriate code and label. Here, we take a look at the top five takeaways from the document: 1.

pharmaphorum
MARCH 30, 2021
Such interventions take the need for robust value claims to a completely different level, with important implications for the evidence companies need to collect, and how they collect and communicate it. There are a number of drivers behind the need to rethink value evidence and value communication. Changes are afoot – what to look for.

pharmaphorum
DECEMBER 7, 2020
Wokingham, United Kingdom — 7 December 2020 — PRISYM ID, a leading provider of regulated content and label management solutions for the life sciences sector, announced today that PRISYM 360 version 1.10 PRISYM 360 and SAP technologies communicate through a web service using standard SAP components with no intermediate stages.

The Checkup by Singlecare
APRIL 18, 2025
Its also prescribed off-label for other types of nerve pain, diabetic neuropathy, mood disorders, and alcohol use disorder. It doesnt require immediate medical attention, but its important to communicate any side effects to a healthcare provider so they can address them.

Pharma Marketing Network
AUGUST 9, 2023
Navigating these regulatory challenges is essential to ensure compliance, maintain trust, and effectively communicate the benefits and risks of pharmaceutical products. Develop Clear and Balanced Messaging Effective communication is at the heart of pharmaceutical marketing. Keep thorough records of approvals for future reference.

The Checkup by Singlecare
MARCH 31, 2024
Patients should carefully review medication labels and consult healthcare professionals to understand potential side effects before driving. In addition, you should read labels carefully, understand medication interactions, and plan for safe transportation. Openly share your concerns and experiences to receive personalized guidance.

The Checkup by Singlecare
MAY 23, 2023
Though most commonly prescribed to treat depression, it is sometimes prescribed off-label for attention-deficit/hyperactivity disorder (ADHD) or (the 12-hour extended-release form) smoking cessation. It is also sometimes prescribed off-label for ADHD or smoking cessation. Not all antidepressants work the same way.

Pharmafile
FEBRUARY 28, 2023
Johnson & Johnson (J&J)’s Janssen-Cilag unit has announced that its fixed-dose combination drug Akeega has been recommended for approval by the EMA’s Committee for Medicinal Products for Human Use (CHMP) for patients with metastatic castration-resistant prostate cancer (mCRPC), but that it should be limited to patients with BRCA1/2 mutations. (..)

pharmaphorum
OCTOBER 12, 2018
The development of software applications that are available with a prescription took a major step forward last year with the first FDA approval for a mobile medical application with both a safety and efficacy label.

The FDA Law Blog
NOVEMBER 29, 2023
Considerations include transparency regarding the data used to develop the change, comprehensive testing of the change, characterizing the performance of the device before and after the change, and plans in place for ongoing monitoring of device performance and communication of any unexpected changes in performance.

The FDA Law Blog
AUGUST 11, 2024
The approved labeling for AUVI-Q includes warnings and precautions regarding emergency treatment, injection-related complications, serious infections at the injection site, allergic reactions associated with sulfite, and disease interactions. The Mirati letter deals with much more nuanced issues than the letter to kaleo.
pharmaphorum
SEPTEMBER 29, 2022
Effective communication is vital for addressing […] barriers, and language is one of the key tools we use to communicate. Despite this, non-inclusive words, phrases, and labels are often used in scientific publications and clinical trial protocols. Barriers to health. Equitable health.

Pharma Marketing Network
MAY 2, 2025
Healthcare professionals (HCPs), too, are inundated with branded communications. A trusted brand is one that delivers value beyond the product label. Patient-Centric Communication Todays healthcare audience expects to be spoken tonot talked at. It must be rooted in authenticity and clarity.

Digital Pharmacist
DECEMBER 6, 2022
Properly organizing medication bottles in a systematic manner where the label is visible may be a good idea. Communicate with patients to record any known drug allergies in the system. It’s important to ensure that they are either stored away from each other or that there is a distinct way to differentiate them. Verify Orders.

SingleCare
OCTOBER 7, 2019
Meaning, the vast majority of patients may not be able to read and fully understand their prescription labels or follow-up instructions. The AHRQ also offers Health Literacy Tools for Use in Pharmacies , which includes info to increase pharmacy staffs understanding of health literacy and how to communicate more effectively with patients.
pharmaphorum
SEPTEMBER 6, 2022
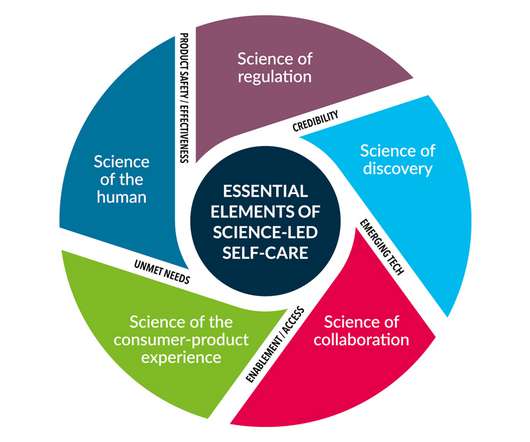
The first principle, labelled the “Science of the Human”, asserts that self-care products should be rooted in a thorough understanding of human biology and medical insights, while the second, the “Science of Regulation”, calls for independent regulation.

The Checkup by Singlecare
JUNE 5, 2025
Like other angiotensin-converting enzyme (ACE) inhibitors, this medication is also used off-label to treat and prevent diabetic kidney disease. But it never hurts to communicate your needs. Without insurance, brand-name Vasotec costs about $1,025 for 30, 20 mg tablets.

Pharmaceutical Technology
JANUARY 24, 2023
The multi-centre, open-label, single-arm clinical trial has been designed to evaluate the efficacy, tolerability, and safety of a single dose of NGN-401 delivered using a one-time ICV procedure. Neurogene stated that the FDA IND clearance allows it to commence a Phase II/II trial of NGN-401 in female paediatric Rett syndrome patients.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content