Q&A: How IQVIA Keeps Pharmacists Engaged with US Prescription Drug Use
Drug Topics
JULY 14, 2025
Often those very high-end medicines are for very few people.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Drug Topics
JULY 14, 2025
Often those very high-end medicines are for very few people.

Pharmacy Times
JULY 16, 2025
14,15 Lung Cancer Tarlatamab Significantly Improves Survival in Patients With Small Cell Lung Cancer Post Progression Tarlatamab has shown promising efficacy in relapsed SCLC based on the initial single-arm trial findings, leading to an FDA approval in 2024, but challenges with administration have limited its widespread adoption.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pharmacy Times
JUNE 9, 2025
SHOW MORE Nivolumab (Opdivo) and chemotherapy demonstrated an overall survival benefit at 5 years, affirming its role as a standard of care option. 1,2 Nivolumab and chemotherapy is a standard of care option for resectable non-small cell lung cancer.

Pharmacy Times
JUNE 27, 2025
SHOW MORE The FDA approved acalabrutinib as a frontline option for untreated mantle cell lymphoma. Mann School of Pharmacy and Pharmaceutical Sciences. For this application, the FDA collaborated with the Australian Therapeutic Goods Administration, Health Canada, and Switzerland’s Swissmedic.
Pharmacy Times
JUNE 9, 2025
doi:10.1056/NEJMoa1901963 Newsletter Stay informed on drug updates, treatment guidelines, and pharmacy practice trends—subscribe to Pharmacy Times for weekly clinical insights. N Engl J Med. 2019;380:2020-2030. Subscribe Now!

Pharmacy Times
JUNE 18, 2025
Martino, PharmD, BCOP, APh Kaitlyn Wells, PharmD Sam Martinez, PharmD, BCOP, BCPS Craig A. Martino, PharmD, BCOP, APh Kaitlyn Wells, PharmD Sam Martinez, PharmD, BCOP, BCPS Craig A.

Pharmacy Times
JUNE 11, 2025
Image Credit: © Chinnapong - stock.adobe.com At the 2025 Oncology Pharmacists Connect (OPC) meeting in Austin, Texas, from June 19 to 20, 2025, an encore presentation from the Pacific Coast Reproductive Society Annual Meeting will highlight findings from a retrospective analysis conducted by Walgreens Specialty Pharmacy.

Pharmacy Times
JUNE 23, 2025
The Orange Book is the reference, and it allows the FDA to declare something [to be] therapeutically equivalent. The FDA Approval Process Within the FDA approval process, the 505(b)(1) new drug application (NDA) submission pathway is the pathway by which new drugs are able to seek approval.

Pharmacy Times
JUNE 17, 2025
News All News FDA Updates Press Releases Media All Videos Digital Detail Independent Corner Insights Interviews MEDcast Medical World News Microsites Peer Exchange Perfect Consult Podcasts Practice Pearls Sponsored Webcast Student Voices Webinars/Webcasts Conferences Conference Coverage Conference Listing Publications Pharmacy Times Pharmacy Practice (..)

Pharmacy Times
JUNE 20, 2025
Blood cancer patients are usually at higher risk due to prior chemotherapy, and maybe we don’t need the same kind of prophylactic medications for solid tumor patients. I think we’re getting to the point now where hematology and oncology both need services that can manage these types of side effects. The infection risks are a little different.

Pharmacy Times
JUNE 12, 2025
The FDA approval process for biologics is lengthier compared with biosimilars. Darkow explained that this is because the FDA mandated that biosimilars must have the same core name as the reference product but must remain identifiable.

Pharmacy Times
JUNE 10, 2025
Meloxicam injection (Xifyrm; Azurity Pharmaceuticals, Inc) received FDA approval for use in adults for the management of moderate-to-severe pain, either alone or in combination with non-steroidal anti-inflammatory drug (NSAID) analgesics. Designing the ideal perioperative pain management plan starts with multimodal analgesia. August 24, 2018.

Pharmacy Times
JULY 9, 2025
Although improved clinical outcomes with the addition of chemotherapy to ICI have been recorded for some patients, there is ongoing research to understand how prognostic factors (eg, smoking history, tumor histology, performance status) can provide some predictive values for patient response to ICI therapy.
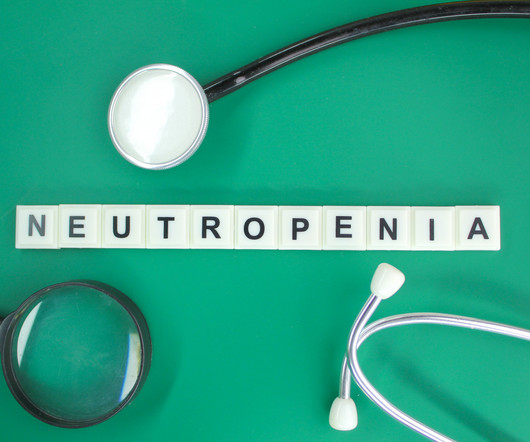
Pharmacy Times
JUNE 16, 2025
Transportation barriers, as a health-related social need, significantly impact FN rates in chemotherapy patients. Febrile neutropenia (FN) remains a serious and common complication of chemotherapy. A 67% FN rate difference between pegfilgrastim and filgrastim was observed in patients with transportation barriers.

Pharmacy Times
JUNE 13, 2025
Vitamin D supplementation increased 25-hydroxyvitamin-D levels significantly, suggesting a potential role in enhancing chemotherapy effectiveness. The study's findings support further research with larger samples to explore vitamin D's impact on chemotherapy and breast cancer remission likelihood.

Pharmacy Times
JUNE 20, 2025
News All News FDA Updates Press Releases Media All Videos Digital Detail Independent Corner Insights Interviews MEDcast Medical World News Microsites Peer Exchange Perfect Consult Podcasts Practice Pearls Sponsored Webcast Student Voices Webinars/Webcasts Conferences Conference Coverage Conference Listing Publications Pharmacy Times Pharmacy Practice (..)
Pharmacy Times
JUNE 19, 2025
2-4 In the United States, the FDA had approved 44 cell therapy products as of March 6, 2025. In October 2024, CAR T therapy axicabtagene ciloleucel (Yescarta; Kite Pharma) received FDA regenerative medicine advanced therapy designation as a first-line treatment for high-risk large B-cell lymphoma. Accessed February 2025. March 6, 2025.

Pharmacy Times
JUNE 9, 2025
link] Newsletter Stay informed on drug updates, treatment guidelines, and pharmacy practice trends—subscribe to Pharmacy Times for weekly clinical insights. Subscribe Now!
Pharmacy Times
JUNE 17, 2025
Conclusions We demonstrated the feasibility of leveraging a complex EHR data source from an integrated health system to study the impact of dose modifications on clinical outcomes for self-administered chemotherapy. Nearly half of the patients with CML on imatinib experienced dosage modifications in this study.

Pharmacy Times
JUNE 25, 2025
Yes, we currently have antibody-drug conjugates that are conjugated to a cytotoxic chemotherapy. I know the FDA is very interested in using real-world data to help inform and confirm some of the benefits that we are seeing in trials. We look at ADCs. Unfortunately, these drugs are quite toxic.
Pharmacy Times
JUNE 11, 2025
The FDA approved taletrectinib (Ibtrozi; Nuavtion Bio Inc) for the treatment of adult patients with locally advanced or metastatic ROS1-positive (ROS1+) non–small cell lung cancer (NSCLC). It demonstrated high response rates with durable benefit and intracranial activity and was well-tolerated among patients, according to the FDA.

Pharmacy Times
JUNE 27, 2025
After learning of his diagnosis, our relationship shifted from small talk at school events to detailed discussions in an infusion center setting about chemotherapy, immunotherapy, clinical trials, alternative medicines, and anticipated adverse effects.

Pharmacy Times
JUNE 9, 2025
Dalton shared her surprise about how variability in manufacturing affects the patient experience, given that some products created using the patient’s cells may not always be usable or up to FDA standards. Presented at: American Society of Health-System Pharmacists 2025 Pharmacy Futures. REFERENCES 1. Updated March 20, 2023.

Pharmacy Times
JUNE 25, 2025
Benmelstobart (TQB2450; Chia Tai Tianqing Pharmaceutical Group Co, Ltd) in combination with chemotherapy followed by sequential combination with anlotinib (Catequentinib; Advenchen Laboratories) led to significant improvements in progression-free survival (PFS), with a manageable safety profile. months versus 7.79 months with tislelizumab.

Pharmacy Times
JULY 14, 2025
SHOW MORE FDA fast-tracks TRE-515 for advanced prostate cancer, combining it with radiation therapy to enhance treatment outcomes and patient care. The FDA has granted fast track designation to TRE-515 (Trethera Corporation), a novel drug, in combination with radiation therapy for the treatment of prostate cancer.
Pharmacy Times
JUNE 18, 2025
News All News FDA Updates Press Releases Media All Videos Digital Detail Independent Corner Insights Interviews MEDcast Medical World News Microsites Peer Exchange Perfect Consult Podcasts Practice Pearls Sponsored Webcast Student Voices Webinars/Webcasts Conferences Conference Coverage Conference Listing Publications Pharmacy Times Pharmacy Practice (..)

Pharmacy Times
JUNE 18, 2025
Mok : Right now, we have a lot of chemotherapy being used, but there are many more novel treatments being studied, especially in earlier lines of treatment. Bispecifics are moving up in the lymphoma space, and these newer therapies may have some synergistic effects with chemotherapy. REFERENCES 1. Updated August 18, 2021.

Pharmacy Times
JULY 3, 2025
So we are licensed by the medical board and also by the board of pharmacy. So we'll send the prescriptions that they need to our specialty pharmacy and then get those meds and bring them to the patient prior to them ever coming to transplant and go, “Okay, these are the meds you're going to take.
Pharmacy Times
JUNE 19, 2025
1 Biomarker testing is relatively new in the treatment landscape for NSCLC but has proven incredibly important for personalizing patient care, transforming from a generic chemotherapy approach to a highly personalized, genomically driven treatment strategy. For advanced disease, chemotherapy was the standard.

Pharmacy Times
JUNE 25, 2025
link] Newsletter Stay informed on drug updates, treatment guidelines, and pharmacy practice trends—subscribe to Pharmacy Times for weekly clinical insights. Subscribe Now!
Pharmacy Times
JUNE 18, 2025
News All News FDA Updates Press Releases Media All Videos Digital Detail Independent Corner Insights Interviews MEDcast Medical World News Microsites Peer Exchange Perfect Consult Podcasts Practice Pearls Sponsored Webcast Student Voices Webinars/Webcasts Conferences Conference Coverage Conference Listing Publications Pharmacy Times Pharmacy Practice (..)

Pharmacy Times
JUNE 6, 2025
News All News FDA Updates Press Releases Media All Videos Digital Detail Independent Corner Insights Interviews MEDcast Medical World News Microsites Peer Exchange Perfect Consult Podcasts Practice Pearls Sponsored Webcast Student Voices Webinars/Webcasts Conferences Conference Coverage Conference Listing Publications Pharmacy Times Pharmacy Practice (..)
Pharmacy Times
JUNE 17, 2025
link] Newsletter Stay informed on drug updates, treatment guidelines, and pharmacy practice trends—subscribe to Pharmacy Times for weekly clinical insights. Subscribe Now! Martino, PharmD, BCOP, APh Kaitlyn Wells, PharmD Sam Martinez, PharmD, BCOP, BCPS Craig A. Stevens, PharmD, BCPS June 3rd 2025 S2.
Pharmacy Times
JUNE 23, 2025
The FDA's decision represents progress in treating EGFR-mutated NSCLC beyond initial therapy lines. SHOW MORE Datopotamab deruxtecan gains FDA approval, offering new hope for adults with advanced EGFR-mutated non-small cell lung cancer after prior therapies.
Pharmacy Times
JUNE 9, 2025
link] Newsletter Stay informed on drug updates, treatment guidelines, and pharmacy practice trends—subscribe to Pharmacy Times for weekly clinical insights. Subscribe Now!
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content