HHS: Medicare incentives, penalties for hospitals could help stem drug shortages
Fierce Healthcare
APRIL 3, 2024
The program, and its complementary effort targeting manufacturers, would require new authorities from Congress.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

 authors resilience
authors resilience 
Fierce Healthcare
APRIL 3, 2024
The program, and its complementary effort targeting manufacturers, would require new authorities from Congress.

pharmaphorum
OCTOBER 12, 2021
Value-based, person-centred healthcare is the key to system resilience – and now is the time to make it happen. The onset of (the pandemic) presents rare momentum to realise ambitious reforms and establish a new paradigm for defining and building health system resilience that will also answer the long-term health challenges Europe faces.”.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

European Pharmaceutical Review
SEPTEMBER 29, 2023
The system can function uninterrupted and operator free, the authors stated. No chemical verification is done by default, due to time constraints and a limit in the number of qualified personnel available, the authors wrote. Current quality control challenges in pharmaceutical packaging Flores et al. In comparison, only 97.4

ISPE
JULY 26, 2023
The 2023 ISPE Annual Meeting & Expo comes at a crucial time, addressing Supply Chain Resiliency and Sustainability to provide the industry with essential insights, strategies, and best practices. By coming together at the 2023 ISPE Annual Meeting & Expo, pharmaceutical professionals can collaboratively Shape the Future of Pharma™.

The FDA Law Blog
APRIL 8, 2024
With the COVID-19 pandemic in the not-so-distant past, we trust that a recitation of the importance of a resilient supply chain is not needed here. HHS would then use MRAP scores in the proposed Hospital Resilient Supply Program (HRSP). While DEA does have the regulatory authority to adjust quotas, such actions are not immediate.

European Pharmaceutical Review
APRIL 10, 2024
A novel routine nuclease testing regimen developed to ensure resilience against nuclease contamination can be utilised as part a laboratory’s quality management system (QMS), a paper reports. The regimen may be applied as a quality indicator fulfilling certain ISO quality control criteria, and offers “documented, systematic feedback”.

European Pharmaceutical Review
MAY 26, 2023
Manufacturing The manufacturing arm of the UK’s life sciences sector is also set for a funding boost thanks to three new pots to bolster the country’s health resilience. A Biomanufacturing Fund worth up to £38 million in new funding has been announced to incentivise investment and improve the UK’s resilience to any future pandemics.

The FDA Law Blog
APRIL 2, 2024
In addition to those initiatives relating to generic competition, the FY25 Legislative Proposals is filled with requests for other legislative authority. FDA also asks for recall authority over drugs—both human and animal—to remove violative drug products from market “more quickly”.

pharmaphorum
AUGUST 12, 2020
Author and journalist John-Manuel Andriote has spent much of his career writing about the impact of HIV/AIDS – a job that became much more personal when he himself was diagnosed in 2005. He tells pharmaphorum about the importance of resilience in coping with a serious illness. Resilience’ is the key term that defines the book for John.

Pharmaceutical Technology
MAY 18, 2023
Amongst the recommendations are calls for informing national competent authorities of impending shortages, creating shortage prevention protocols, designing multinational supply chains to increase resilience, and communication lines between stakeholders and supply chains.
European Pharmaceutical Review
MAY 18, 2023
Key recommendations include: Informing national competent authorities of potential or actual medicine shortages as early as possible and providing detailed information to better predict the possible impact and implement preventive measures establishing robust shortage prevention and shortage management plans optimising pharmaceutical quality systems (..)

European Pharmaceutical Review
NOVEMBER 15, 2022
According to IGBA, the World Health Organization ( WHO) Biosimilars Guideline has been a useful tool for health authorities to boost international regulatory convergence and consistency of terminology when evaluating biosimilars, by approving quality, safe and efficacious biosimilar medicines for patients who otherwise would have lacked access.

European Pharmaceutical Review
JANUARY 16, 2023
Interpharma, Switzerland’s research-based pharmaceutical industry association stated the agreement “…will strengthen the resilience of supply chains and therefore improve the security of supply.”. Good Manufacturing Practice for pharmaceutical drugs.

European Pharmaceutical Review
JULY 21, 2023
There are four ways: Establish strong regulatory partnerships: Collaborate with regulatory authorities to establish clear guidelines, harmonise regulations, and streamline processes across different regions and countries. Going forward, it is imperative for companies to invest in constructing more resilient supply networks.

pharmaphorum
NOVEMBER 26, 2020
As part of our EU Leader series, Christian Pawlu, head of strategy, portfolio and BD&L at Sandoz, told us about how securing supply in a time of crisis will ensure future access, build resilience, and transform relationships. Resilient future. I think the balance we need to keep in mind is cost, quality, and resilient supply.

European Pharmaceutical Review
AUGUST 2, 2023
Authored by experts at Amgen, the paper asserted that delays associated with off-line analytical testing can hinder the speed of process development. Real-time monitoring and control of the biomanufacturing processes through product quality insights continues to be a key area of focus in the biopharmaceutical industry, the paper reported.

ISPE
SEPTEMBER 8, 2023
Download the ISPE Drug Shortages Prevention Model REPORT ON ISPE WORK TO SUPPORT ESTABLISHMENT OF THE EUROPEAN UNION HEALTH EMERGENCY PREPAREDNESS AND RESPONSE AUTHORITY (HERA) The EU HERA was launched as a new European Commission Directorate-General with a mission to prevent, detect, and rapidly respond to health emergencies.
pharmaphorum
JANUARY 18, 2023
In 2016, scientists behind a study called the Resilience Project analysed genetic data from 589,000+ people and found 13 adults who carried genetic variants that should have resulted in serious – even deadly – childhood disease, but who were apparently healthy. About the author. Giving participants something in return.

pharmaphorum
JUNE 11, 2021
Therefore, it is up to individual organisations to foster a culture of resilience and vigilance, especially in those areas that work with sensitive patient and trial information, going well beyond compliance to consider what assets are most valuable for trade in the dark web. About the author. Executive leadership and collaboration.
Hospital Pharmacy Europe
NOVEMBER 20, 2023
Professor Cátia Caneiras, co-chair of the FIP commission on AMR and co-author of the report, added: ‘The time to act is now, and by equipping the pharmaceutical workforce, we can collectively forge a more resilient future against the threat of AMR.

pharmaphorum
JANUARY 18, 2022
Collaborations have become the only way for some companies to show business resilience during this digital acceleration. Previously uncommon collaborations or less expansive partnerships have started to form between companies, especially as digital transformation has become a fundamental necessity for business resilience.

pharmaphorum
DECEMBER 10, 2021
Working in different roles and projects within and outskirts of Orion Pharma he’s known as innovative, resilient and determined in everything he does. About the author. Sammeli Liikkanen is a Pharma and Healthcare professional with a strong flavour of digitalisation in his background.

pharmaphorum
SEPTEMBER 9, 2022
“With its heavy reliance on natural resources for product development and production, and complex supply chains with large carbon footprints, the pharmaceutical industry has been highlighted as one of the biggest contributors to global emissions,” said the authors of a whitepaper from climate intelligence specialists, Cervest. Resilience.

European Pharmaceutical Review
JULY 15, 2022
Resiliency and renewables. With a comprehensive energy strategy, pharmaceutical businesses can reduce carbon emissions, maintain resilience, predict future energy costs and enhance ESG performance” Going green is not without its challenges. About the author. Conclusions.

pharmaphorum
OCTOBER 8, 2021
Janssen UK & Ireland’s managing director Gaëtan Leblay discusses the importance of maintaining the spotlight on our mental health post-pandemic, and why we must hone our mental resilience for possible future health crises. Can we be mentally resilient in the future? About the author. Technology and data in mental well-care.
pharmaphorum
JANUARY 26, 2021
Leading experts in health care provision, patient representation, policy and industry from across Europe – France, Germany, Italy, Spain, the United Kingdom – met regularly to discuss the current global healthcare environment and plan for the future in improving the resilience of lung cancer services.
pharmaphorum
NOVEMBER 10, 2022
Preventive and diagnostic care is key to building more inclusive and resilient systems and procedures for the healthcare sector, but we have a long way to go before this vision becomes reality. Resilient health systems have prevention at their core, and building capacity to this end is key, not simply for routine care.

pharmaphorum
DECEMBER 20, 2022
However, this rapidly-built infrastructure often fell short in terms of long-term sustainability and resiliency. About the author. The post Transforming healthcare through sustainable and resilient data infrastructure appeared first on.
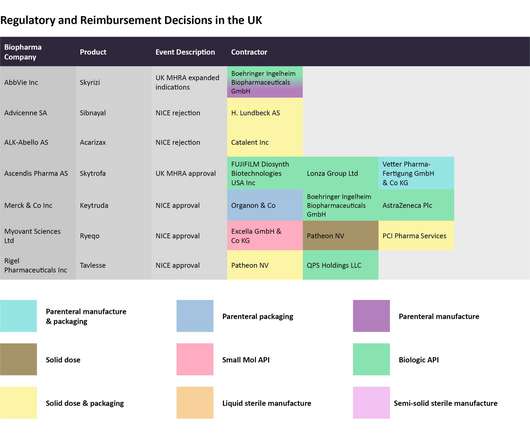
Pharmaceutical Technology
DECEMBER 12, 2022
The European regulator extended Comirnaty’s authorized use to those between the ages of six months and four years. Aldevron LLC, Lonza Group Ltd, National Resilience Inc, and Laboratorios Farmaceuticos Rovi SA are manufacturing the biologic API for the vaccine. Regulatory and reimbursement decisions in the UK.

pharmaphorum
SEPTEMBER 28, 2022
What we saw from the Pre-Cert pilot is that companies that invest in infrastructure around continuous integration and continuous deployment not only have more resilience to changes in terms of the updates that they push, …, but they are also able to fix problems faster when they happen,” O’Leary said.

pharmaphorum
DECEMBER 21, 2022
Emotion – creating an open dialogue between people to boost their understanding of (and resilience to) sustainability. About the author. Mission – embedding purpose and upholding ethics across every area of a business. Innovation – using technology to solve problems without creating harmful side-effects.

The People's Pharmacy
MARCH 30, 2023
During the COVID-19 pandemic, we could all see big differences in who got sick and who seemed more resilient. Dr. Chutkan is the author of the digestive health books Gutbliss , The Microbiome Solution , The Bloat Cure and The Anti-Viral Gut: Tackling Pathogens from the Inside Out. You will find this show well worth your time!

pharmaphorum
NOVEMBER 25, 2020
From streel-level WhatsApp groups to locality-wide delivery of medicines, the benefits of community resilience have been recognised at the highest levels of government, while its link to digital health and power shifting to communities has been made. About the author. Creating health alongside prevention and treatment.
pharmaphorum
JUNE 28, 2022
In the future, the entire global health system will need to change to become more resilient, which requires many individual changes but can be broken down it smaller, logical actions that have outsized outcomes. Resilient national systems: To strengthen a critical foundation for global pandemic preparedness and response.

pharmaphorum
JULY 19, 2021
America’s biopharmaceutical industry says it is “ready to do its part” in building a stronger, more resilient, affordable, and equitable health care system for all. Innovation only makes a difference if what we create can help the people who need it,” said the authors. About the author. Read the full report here.

pharmaphorum
JULY 7, 2021
Lastly, the Vision will also help us to make the UK more resilient to the next pandemic, with a plan to boost our manufacturing base and attract a skilled workforce to make this possible. About the author. The post A life sciences vision to deliver health, wealth and resilience for the UK appeared first on.
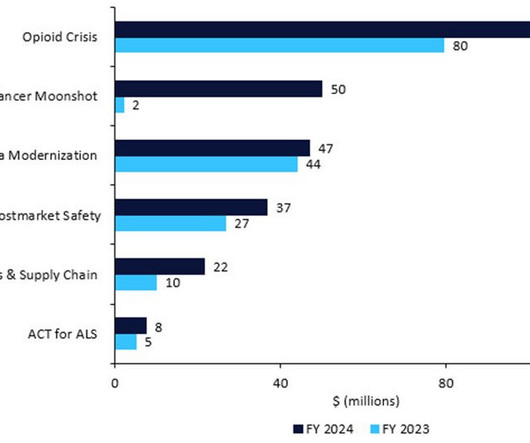
Pharmaceutical Technology
MARCH 30, 2023
Recall powers for all drugs: The budget reveals legislative as well as spending plans; the agency wants to expand its authority to recall any drug. Device shortages and supply chain: +$11.6m, for a total of $21.6m, for the Resilient Supply Chain and Shortages Program for medical devices. ACT for ALS : +$2.5m for a total of $7.5m

pharmaphorum
DECEMBER 9, 2021
The key elements to achieve this rely on being patient-focused, innovation-focused, and forward-looking,” said the authors, adding that Britain must remain an “effective player on the main stage”. “The ABPI believes that there are several ways in which the UK can progress towards an internationally competitive regulatory framework.

pharmaphorum
MAY 16, 2022
USAID is considering these coordination and integration opportunities as part of how it responds not just to COVID-19 but more broadly to meet current needs and ensure countries are made more resilient and strengthened for future health shocks. About the author. The Future of Information-Sharing in Humanitarian Emergencies.

pharmaphorum
AUGUST 12, 2022
That’s a perfectly reasonable response – domestic production is much more resilient. Not only will this make domestic drug supplies resilient in an uncertain future. About the author. But reshoring is also a huge challenge given the economic, regulatory, and logistical obstacles involved. The case for inshoring is clear.

pharmaphorum
JANUARY 26, 2023
Next year will likely see the first commercial projects employing precision breeding, particularly focused on developing resilient, higher-yield and drought resistant crops. About the author Neil Ward is VP and general manager for Europe, Middle East, and Africa at PacBio.

European Pharmaceutical Review
NOVEMBER 2, 2023
One recommendation sought the optimisation of pharmaceutical quality systems and increased resilience of complex, multinational supply chains. However, in recent months, a range of European organisations have made progress in addressing medicine shortages, through new actions, working groups and more.

European Pharmaceutical Review
APRIL 21, 2023
About the author Dorothea Baltruks is a Research Associate at the Centre for Planetary Health Policy (CPHP) where she focuses on the transformation of the German health system towards climate resilience and sustainability. The 2022 Europe report of the Lancet Countdown on health and climate change: Towards a climate resilient future.
pharmaphorum
JULY 22, 2021
We’ve had strong and consistent leadership and high-level management support throughout the entire pandemic who have led by example, encouraging us to be agile and resilient. Celltrion is also in discussion with regulatory authorities in other regions including the U.S.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?

Let's personalize your content