CMO Moves: Regulatory catalysts for drug manufacturing- February
Pharmaceutical Technology
FEBRUARY 27, 2023
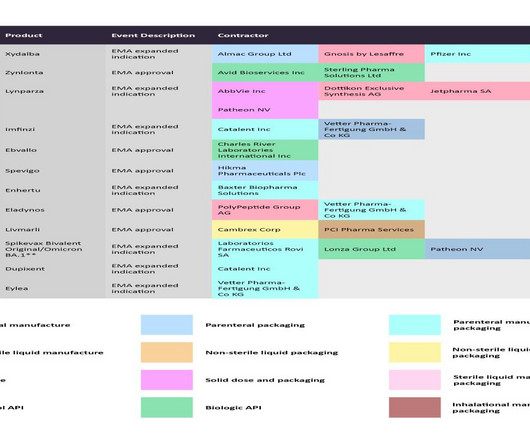
Towards the end of 2022, the EC also awarded a conditional marketing authorization for another ADC — Sobi’s and ADC Therapeutics’ Zynlonta (loncastuximab tesirine) to treat relapsed or refractory (r/r) diffuse large B-cell lymphoma (DLBCL). Avid Bioservices and Sterling Pharma Solutions are manufacturing Zynlonta’s biological API.








Let's personalize your content