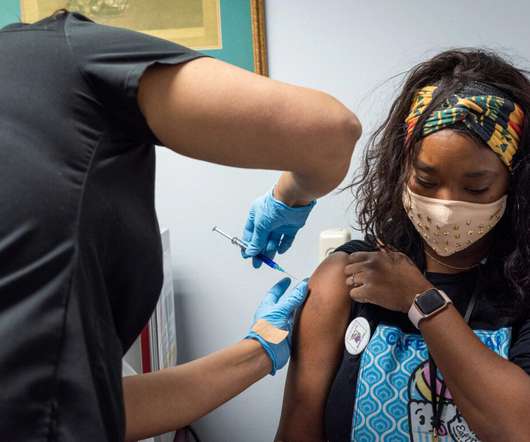
COVID-19 Vaccine Safe for Children Who Had Previous MIS-C Diagnosis
Drug Topics
JANUARY 9, 2023
Patients with a history of multisystem inflammatory syndrome in children (MIS-C) completed a questionnaire about adverse reactions following COVID-19 vaccination, and no serious adverse events were recorded.






























Let's personalize your content