Remote Acceptance Testing of Automation Projects
ISPE
OCTOBER 28, 2022
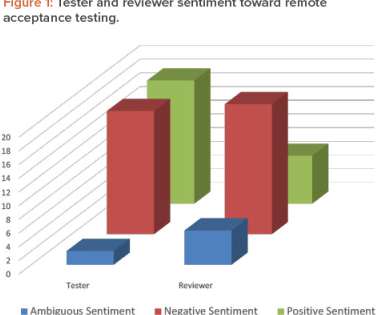
Before the COVID-19 pandemic, it was unthinkable for a system integrator to suggest remotely conducting an acceptance test for an automation project. Before the COVID-19 pandemic, acceptance testing of automation projects was conducted in person, with multiple subject matter experts attending. is used as a guide.













Let's personalize your content