STAT+: Pharmalittle: FDA approves Pfizer’s RSV vaccine for newborns; Boehringer sues over IRA
STAT
AUGUST 22, 2023
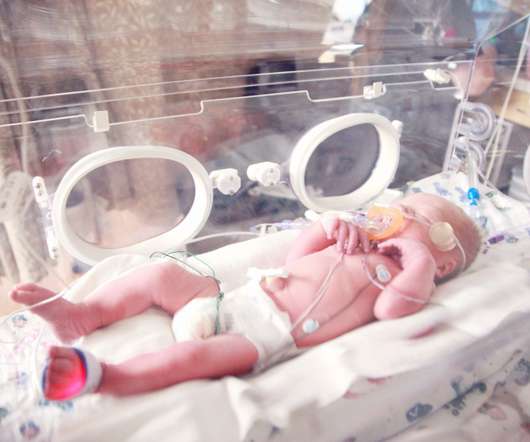
The Food and Drug Administration approved a Pfizer vaccine that aims to protect newborns against RSV by vaccinating pregnant people, STAT tells us. The vaccine, marketed as Abrysvo, previously won approval for adults over the age of 60. Continue to STAT+ to read the full story…













Let's personalize your content