FDA $7bn plans for 2024: disclose contract manufacturers, restart Cancer Moonshot
Pharmaceutical Technology
MARCH 30, 2023
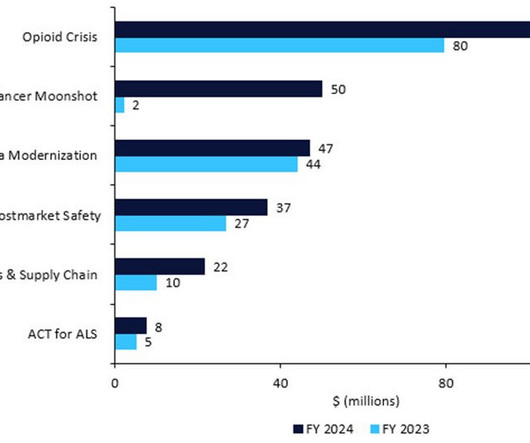
Table 1: Medical product (drugs, devices, and diagnostics) safety budget, 2023 vs. 2024 Source: FDA; GlobalData © GlobalData Whether the proposal passes, and with what alterations, will depend on US Congress. This is made up of $2.1bn in direct funding and $2.4bn in user fees.












Let's personalize your content