FDA approves first treatment for geographic atrophy
European Pharmaceutical Review
FEBRUARY 21, 2023
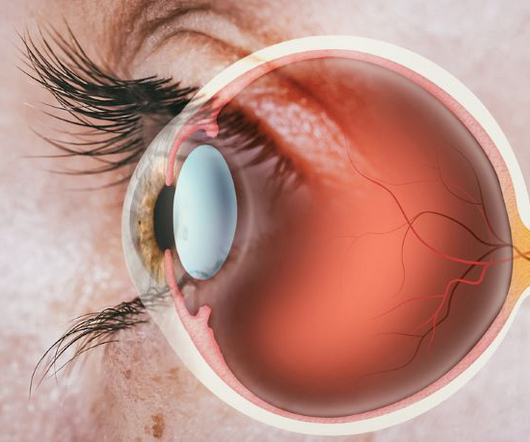
SYFOVRE (pegcetacoplan injection) is the first and only treatment approved by the US Food and Drug Administration (FDA) for geographic atrophy (GA), a leading cause of blindness. A marketing authorisation application (MAA) is under review by the European Medicines Agency (EMA) with a regulatory decision expected in early 2024.
















Let's personalize your content