FDA Approves Updated Label for Altuviiio for Effective Bleed Protection in Children Younger than 12 Years
Drug Topics
MAY 13, 2024
Altuviiio was first granted approval by the FDA in February 2023.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Drug Topics
MAY 13, 2024
Altuviiio was first granted approval by the FDA in February 2023.

pharmaphorum
NOVEMBER 17, 2023
AZ first to AKT finish line, but FDA clears narrow label Phil.Taylor Fri, 17/11/2023 - 09:46 Bookmark this
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pharmaceutical Technology
MARCH 22, 2023
The US Food and Drug Administration (FDA) has provided guidance for the use of RFID in the drug supply chain and to standardise the data format. In the US Drug Quality and Security Act (DSCSA), the FDA mandates that manufacturers and trading partners should have full interoperable electronic track and trace systems in place by November 2023.
Pharmacy Times
MAY 13, 2024
Antihemophilic factor (recombinant) Fc-VWF-XTEN fusion protein-ehtl was initally approved in February 2023 for adults and children with hemophilia A for prophylaxis and on-demand treatment to control bleeding.

The Checkup by Singlecare
NOVEMBER 9, 2023
Food and Drug Administration (FDA) just approved Zepbound (tirzepatide) for chronic weight management. The injectable medication is a new version of Eli Lilly’s Mounjaro, which is approved by the FDA to control blood sugar in people with Type 2 diabetes. Zepbound, on the other hand, has been FDA-approved for weight loss.

The FDA Law Blog
DECEMBER 8, 2022
Food and Drug Administration (FDA) issued two guidance documents, one draft and one final, on food allergen labeling requirements. 1, 2023; The applicability of food allergen labeling requirements to specific products (e.g., 1, 2023; The applicability of food allergen labeling requirements to specific products (e.g.,
European Pharmaceutical Review
OCTOBER 18, 2023
The US Food and Drug Administration (FDA) has approved XPHOZAH ® (tenapanor), the first and only phosphate absorption inhibitor. XPHOZAH is expected to be available to eligible patients in the US in November 2023.

IDStewardship
JANUARY 13, 2025
In case you are wondering, the FDA label does not list any hepatic dose adjustments. 2023 Jan 6;76(1):78-88. 2023 Jan 6;76(1):66-77. 2023 Sep 18;77(6):937. The absence of renal dose adjustments may be attributed to sulopenem dual excretion pathways, with elimination occurring through both feces and urine.

European Pharmaceutical Review
OCTOBER 25, 2023
The US Food and Drug Administration (FDA) has approved a new therapy for adults with relapsed or refractory (R/R) myelodysplastic syndromes (MDS) with an isocitrate dehydrogenase-1 (IDH1) mutation, a rare type of blood cancer. The post FDA approves new therapy for myelodysplastic syndromes appeared first on European Pharmaceutical Review.

Pharmaceutical Technology
APRIL 17, 2023
On 14 April 2023, experts from the US Food and Drug Administration’s (FDA) Advisory Committee (AdCom) voted largely in favour of the potential approval of Otsuka’ s and Lundbeck Pharmaceuticals’ Rexulti for the treatment of agitation associated with Alzheimer’s dementia (AAD). Rexulti is an atypical antipsychotic.

Pharmaceutical Technology
MARCH 2, 2023
The US Food and Drug Administration (FDA) has approved Reata Pharmaceuticals ’ oral, once-daily medication SKYCLARYS (omaveloxolone) to treat Friedreich’s ataxia patients. There are three more drug candidates with major trial readouts that are expected in 2023.
Pharmaceutical Technology
APRIL 17, 2023
The US Food and Drug Administration (FDA) has approved an update to the indications and usage section of Horizon Therapeutics ’ Tepezza (teprotumumab-trbw) label to specify its use to treat thyroid eye disease (TED) patients regardless of disease activity or duration. The FDA’s approval was granted in January 2020.

European Pharmaceutical Review
JANUARY 25, 2024
Draft guidance published by the US Food and Drug Administration (FDA) in December 2023, discussed quality considerations for topical ophthalmic drug products, including key considerations for extractables and leachables (E&L) testing. This document is revised from a version published in October 2023.

pharmaphorum
AUGUST 21, 2022
Shares in Axsome Therapeutics have rocketed on FDA approval of its depression therapy Auvelity (formerly AXS-05) – a year after its approval was held up by the regulator. However, it has a broader label as unlike J&J’s drug it is indicated for use in previously-untreated MDD. Photo by Sydney Sims on Unsplash.
European Pharmaceutical Review
FEBRUARY 7, 2023
The US Food and Drug Administration (FDA) has granted Regenerative Medicine Advanced Therapy (RMAT) designation to RP-A501, an adeno-associated virus (AAV)-based gene therapy for Danon disease. “RP-A501 RMAT designation will provide the benefits of added intensive FDA guidance and expedited review through the programme’s development.

The FDA Law Blog
JANUARY 2, 2023
Hosted by American Conference Institute, the FDA Boot Camp returns for its 40th iteration with the continued intent of providing an essential working knowledge of core FDA concepts, and real-world examples that will help you to excel in your everyday practices. Labeling in the drug and biologics approval process.

pharmaphorum
SEPTEMBER 30, 2022
Sarepta Therapeutics has followed through on its promise to file for accelerated approval of its gene therapy SRP-9001 for Duchenne muscular dystrophy (DMD), as it aims for a launch in the middle of 2023. The post Sarepta files Duchenne muscular dystrophy gene therapy with FDA appeared first on. point NSAA improvement versus a 0.7-point
Pharmaceutical Technology
MAY 2, 2023
The US Food and Drug Administration (FDA) has accepted the supplemental biologics licence application submitted by Bristol Myers Squibb for Reblozyl (luspatercept-aamt) as a first-line treatment of anaemia in adults with lower-risk myelodysplastic syndromes (MDS).

The FDA Law Blog
MAY 23, 2023
Food and Drug Administration (FDA) released a draft update to its Compliance Policy Guide (CPG) for FDA staff on the Agency’s enforcement of major food allergen labeling and cross-contact. The draft CPG directs FDA field staff to examine possible food product adulteration due to labeling related to allergen cross-contact.

The FDA Law Blog
AUGUST 17, 2023
Tobolowsky — In January 2023, Vanda Pharmaceuticals, Inc. FDA-2023-P-0313 and FDA-2023-P-0344 ) regarding its product Hetlioz (tasimelteon). FDA regulations, at 21 C.F.R. a)(8)(iv), interpret these provisions to also allow changes due to an aspect of labeling protected by patent or exclusivity.

The Checkup by Singlecare
OCTOBER 4, 2023
That’s why you may have seen people talk about a medication called Vyvanse, which is FDA approved to treat ADHD and binge eating disorder, as a good option for weight loss. In 2015, the FDA approved Vyvanse to be used in the treatment of binge eating disorder. Does Vyvanse cause weight loss?

Pharmaceutical Technology
DECEMBER 4, 2022
There are seven FDA-approved Humira biosimilars, of which Amgen’s Amjevita will be launched in January. Humira is indicated for 10 adult and pediatric conditions, including Crohn’s disease, rheumatoid arthritis, and ulcerative colitis, as per its label. Humira biosimilars became available in the EU in October 2018.

STAT
JANUARY 25, 2024
One person also experienced hypoglycemia in 2023 after injecting a compounded version of Ozempic, said the organization, which represents 55 regional poison centers across the country and works with the U.S. Ozempic and similar diabetes medicines have been increasingly used off label for weight loss.

The FDA Law Blog
APRIL 25, 2023
This provision became effective as of March 29, 2023. It will become part of the “refuse to accept” (RTA) checklist on October 1, 2023. The primary vehicle for FDA to request cybersecurity information in premarket submissions has been guidance documents. Timeline Section 524B became effective on March 29, 2023.

The Checkup by Singlecare
DECEMBER 6, 2023
In 2023, medications like Ozempic , Mounjaro , and Wegovy soared in popularity—so much so that many pharmacies faced a shortage. Read on to see what readers cared about the most in 2023. Whether you’re taking Ozempic for diabetes or off-label for weight loss, you might be curious if it’s safe to mix it with alcohol.

The FDA Law Blog
MARCH 27, 2023
Such codes need to be placed on device labels and packages to allow devices to be easily identified and tracked throughout their lifecycle, except where the rule provided for an exception or alternative. The compliance dates were first published in 2013, and subsequently updated in various guidance documents and regulations published by FDA.

The FDA Law Blog
FEBRUARY 21, 2024
Mullen — More than five years after FDA first announced its plan to harmonize 21 CFR Part 820 with ISO 13485, on February 2, 2024, FDA finally issued the Quality Management System Regulation (QMSR) Final Rule. FDA further retained some definitions in the QSMR. Notably, Part 820 will look different. Revised § 820.3
Pharmaceutical Technology
OCTOBER 31, 2022
The US Food and Drug Administration (FDA) has accepted Outlook Therapeutics’ Biologics License Application (BLA) filing for ONS-5010 / LYTENAVA (bevacizumab-vikg) to treat wet age-related macular degeneration (wet AMD). The regulator has set 29 August 2023 as a Prescription Drug User Fee Act (PDUFA) goal date.

Pharmaceutical Technology
DECEMBER 11, 2023
Novartis is seeking approval for Kisqali as a treatment of early breast cancer, with an FDA application planned by the end of 2023.

The Checkup by Singlecare
DECEMBER 1, 2023
That is 170% of the daily value (DV) recommended by the US Food and Drug Administration (FDA). Department of Agriculture (USDA) requires it to be labeled “farmed” or” wild-caught.” Check the nutrition facts label to find the amount of vitamin D and other nutrients contained in individual products. mcg or 100–144 IU per cup.

IDStewardship
MARCH 12, 2023
BCPS, BCIDP Article Posted 12 March 2023 As a pharmacist serving as a preceptor to students and residents in the area of infectious diseases since 2010, I have always been on the hunt for good infectious diseases journal articles for my learners to read. Authored by: Timothy P. Gauthier, Pharm.D.,
Pharmaceutical Technology
MAY 18, 2023
The US Food and Drug Administration (FDA) has accepted Ardelyx’s resubmission of a new drug application (NDA) for XPHOZAH (tenapanor) to control serum phosphate in adults with chronic kidney disease on dialysis who have had insufficient response or intolerance to a phosphate binder treatment.

pharmaphorum
AUGUST 4, 2021
Pfizer is now only a little behind Lilly, although it is still waiting for the results of a phase 3 open-label study ( ALLEGRO-LT ) looking at long-term safety and efficacy before it will move ahead with its own regulatory filing. If the results are positive, Concert could be ready to file for approval in 2023.

The Checkup by Singlecare
JANUARY 18, 2023
FDA-approved prescription weight-loss options . Food and Drug Administration (FDA) has now approved these prescription weight-loss meds for long-term use : . Off-label weight-loss medications. Sometimes, your provider may recommend another type of medication for off-label use. FDA-approved weight-loss medications.

The FDA Law Blog
JULY 6, 2023
We now see that the proposed rule to “harmonize and modernize” the QSR with ISO13485:2016, creating the new QMSR, is on the Spring 2023 Unified Agenda (see here ). In fact, the priority designation for the final rule is labeled as “economically significant.” According to the Unified Agenda, the proposed rule is in the final rule stage.

The FDA Law Blog
JUNE 27, 2023
Walsh — Last fall, we blogged about the process FDA uses to review allegations of regulatory misconduct against device manufacturers, suggesting greater transparency on the FDA process was needed (see here ). Any comments to the public notice must be submitted by August 11, 2023. This statistic is quite disheartening.

The FDA Law Blog
AUGUST 13, 2023
In the case of contract manufacturers, either the contract manufacturer or the person whose name appears on the label (i.e., For both the registration and listing, FDA describes optional information that FDA would like to get. Comments on the draft guidance may be submitted until Sept.

The Checkup by Singlecare
JANUARY 26, 2024
Approved by the Food and Drug Administration (FDA) in 2017 for blood sugar management, Ozempic has recently gained attention for its weight loss effects. Healthcare professionals prescribe Ozempic off-label for weight management for patients with obesity. It received FDA approval for weight management in June 2021.)
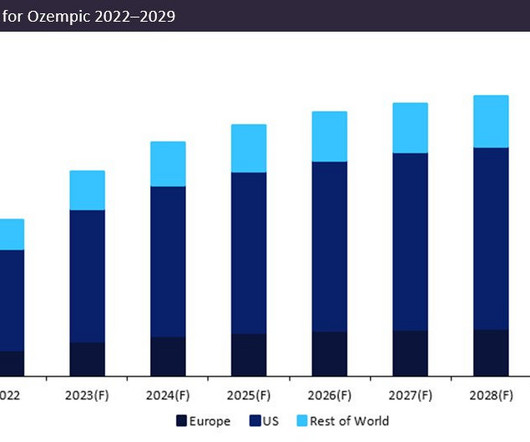
Pharmaceutical Technology
APRIL 28, 2023
Novo Nordisk’s leading drug Ozempic (semaglutide) is forecast to demonstrate a sales growth of 23% in 2023. Ozempic’s forecast 2023 sales of $12.5bn consolidate its position as the dominant market leader, with projected sales in 2023 54% greater than closest competitor Trulicity (dulaglutide) by Eli Lilly, which anticipates sales of $8bn.

The FDA Law Blog
FEBRUARY 28, 2024
This is the third withdrawal of an accelerated approval FDA has performed, following Avastin in 2011 and Makena in 2023. Without commenting on the merits of the decision, it is an interesting window into how FDA may use these new procedures in practice. Tobolowsky & Michelle L.

European Pharmaceutical Review
JULY 31, 2023
In the open label extension study for the trial, patients treated with ZTALMY for at least one year experienced a median 49.6 The EC’s approval of ZTALMY follows a positive opinion issued in May 2023 by the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA).

PharmaShots
MARCH 17, 2023
5-Adapted Bivalent Booster to Treat COVID-19 in Children ≤5 Years Date: Mar 15, 2023 | Tags: Pfizer, BioNTech, Omicron BA.4/BA.5-Adapted 5-Adapted Bivalent Booster to Treat COVID-19 in Children ≤5 Years Date: Mar 15, 2023 | Tags: Pfizer, BioNTech, Omicron BA.4/BA.5-Adapted

PharmaShots
FEBRUARY 3, 2023
4D Molecular Therapeutics Receives the US FDA’s IND Clearance of 4D-150 for the Treatment of Diabetic Macular Edema Date: Feb 03, 2023 | Tags: 4D Molecular Therapeutics, 4D-150, Diabetic Macular Edema, Regulatory, US, FDA, IND AstraZeneca and Amgen Receive the US FDA’s Approval of Tezspire (tezepelumab) for the Treatment of Severe Asthma (..)

The FDA Law Blog
MARCH 24, 2025
Houck In August 2023, the U.S. If rescheduled to schedule III, marijuana businesses would be required to obtain DEA registrations, take initial and biennial inventories of marijuana on-hand, maintain transaction records, file theft and significant loss reports, and label and secure products appropriately. By Larry K.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content