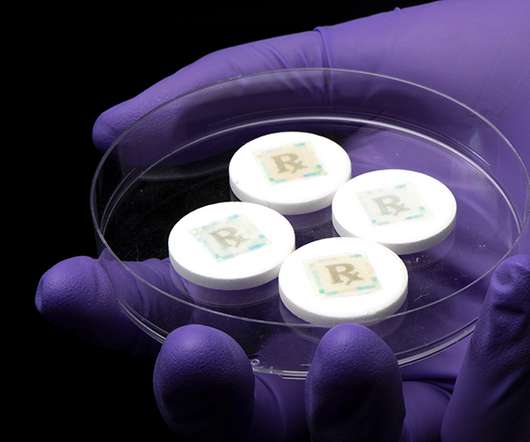
Securing every dose with an edible security technology for safe medicines
European Pharmaceutical Review
AUGUST 23, 2022
On- or in-dose authentication means that a security measure or anticounterfeit feature is integrated with the dosage form itself, offering product verification and traceability embedded into each medicine, rather than on the secondary package” WHO estimates that >50 percent of the drugs for sale on the internet are fake.










Let's personalize your content