End-Of-Life Management for Single-Use Products in Bioproduction
ISPE
MARCH 14, 2023
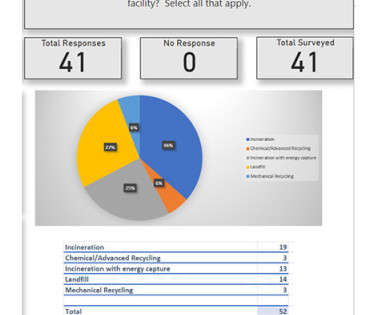
The survey was distributed via an email communications to ISPE members, a blog on ISPE’s website, and LinkedIn postings. This provided impetus for the survey whose goal was to identify users’ current practices and views on what they would like to see happen.










Let's personalize your content