mRNA: A New Era in Biopharmaceutical Contract Manufacturing Market
Roots Analysis
AUGUST 16, 2023
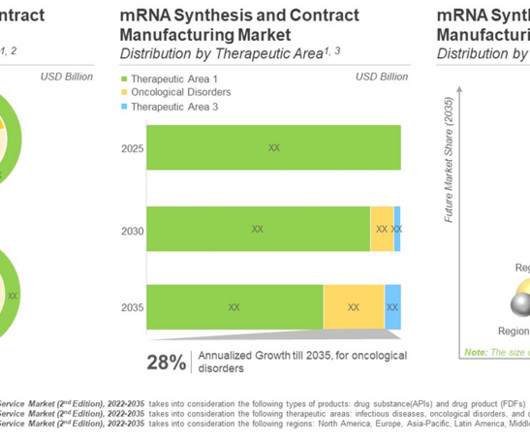
The success of COVID-19 vaccines paved the path for mRNA-based drug products. Safety, efficacy and rapid production of mRNA drives the interest in this domain. Concluding Remarks Currently, dozens of preclinical and clinical reports demonstrating the efficacy of these platforms have been published in the last two years alone.





















Let's personalize your content