PharmaShots Weekly Snapshots (April 24 - 28, 2023)
PharmaShots
APRIL 28, 2023
Difficile Infection Date: Apr 27, 2023 | Tags: Seres Therapeutics, Nestlé Health Science, Vowst, C.

 tag mavacamten
tag mavacamten 
PharmaShots
APRIL 28, 2023
Difficile Infection Date: Apr 27, 2023 | Tags: Seres Therapeutics, Nestlé Health Science, Vowst, C.

PharmaShots
JUNE 2, 2023
Impact Therapeutics Entered into a License and Collaboration Agreement with Eikon Therapeutics to Develop and Commercialize IMP1734 Date: June 02, 2023 | Tags: Impact Therapeutics, Eikon Therapeutics, IMP1734, Pharma, China, Hong Kong, Macau, Taiwan BMS’ Mavacamten Receives the NICE Recommendation for the Treatment of Obstructive Hypertrophic (..)
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

PharmaShots
JUNE 16, 2023
Date: June 12, 2023 | Tags: Novartis, Chinook Therapeutics, Atrasentan, Zigakibart, BION-1301, IgA nephropathy, M&A, ~$3.5B Date: June 12, 2023 | Tags: Novartis, Chinook Therapeutics, Atrasentan, Zigakibart, BION-1301, IgA nephropathy, M&A, ~$3.5B
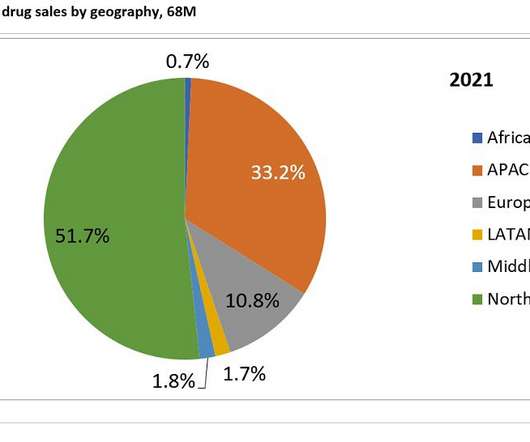
Pharmaceutical Technology
DECEMBER 15, 2022
Based on the previous 7MM report, growth in the cardiomyopathies market was primarily attributed to the market release of Bristol Myers Squibb’s Camzyos (mavacamten) and the upcoming launch of Cytokinetics’ aficamten, both of which are myosin inhibitors indicated for the management of obstructive hypertrophic cardiomyopathy (HCM).
Let's personalize your content