NICE says yes to Novartis’ multiple sclerosis therapy Kesimpta
pharmaphorum
APRIL 20, 2021
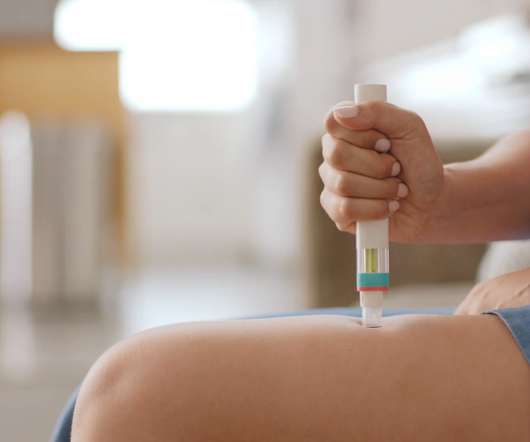
Novartis has secured backing from NICE for its relapsing multiple sclerosis (RMS) therapy Kesimpta in the UK, just two weeks after the drug was approved by the national drugs regulator. . Nearly nine out of ten patients treated with Kesimpta saw no evidence of disease activity in their second year of treatment with the drug. .









Let's personalize your content