Haemophilia drug costing $3.5m per dose has been approved
Pharmafile
NOVEMBER 23, 2022
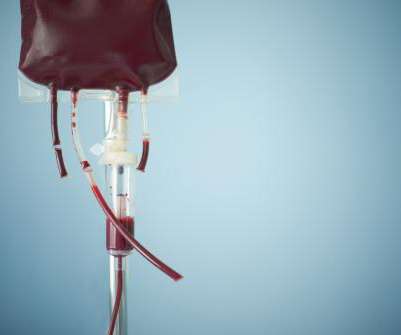
CSL Behring’s haemophilia B gene therapy has recently been approved by US regulators, however the treatment has an enormous price tag. CSL Behring’s Hemgenix is a one-dose treatment which appeared to reduce the number of bleeding events by 54% over the course of a year. Costing $3.5m









Let's personalize your content