Rare Disease Spotlight – tracing the rise of orphan drug designations over almost 40 years
Pharmaceutical Technology
JUNE 29, 2022
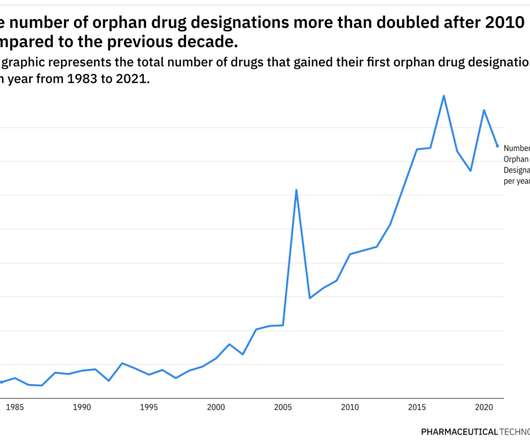
Additionally, pricing and access for rare disease therapies continue to be scrutinized closely. In a series of data-driven deep dives, we will examine how the development of orphan drug therapies has evolved over the years, and use the FDA’s orphan drug program as a barometer of these advances.








Let's personalize your content