7 Problems with Using Borosilicate Glass to Package Drugs
Pharma Mirror
FEBRUARY 18, 2022
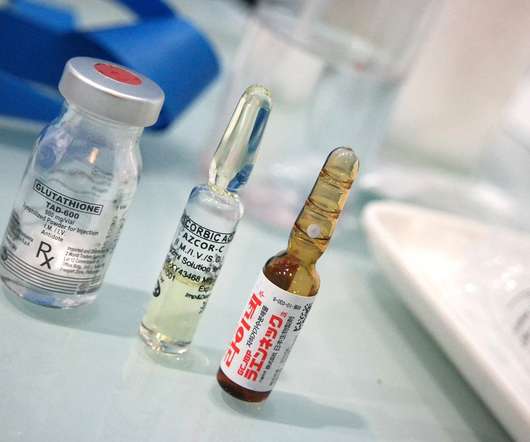
By Christopher Weikart, Chief Scientist, SiO2 Materials Science Even though drugs are meant to save lives, the primary packaging used for delivering drug therapies and vaccines may actually be putting us in danger. The post 7 Problems with Using Borosilicate Glass to Package Drugs appeared first on Pharma Mirror Magazine.













Let's personalize your content